
Thermodynamics: An Engineering Approach
9th Edition
ISBN: 9781260048766
Author: CENGEL
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 8.8, Problem 61P
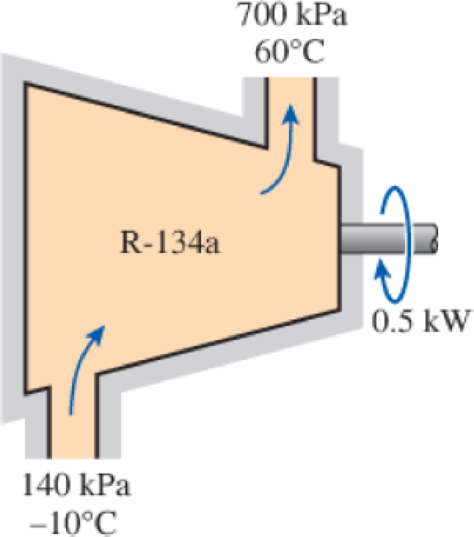
Refrigerant-134a at 140 kPa and −10°C is compressed by an adiabatic 0.5-kW compressor to an exit state of 700 kPa and 60°C. Neglecting the changes in kinetic and potential energies and assuming the surroundings to be at 27°C, determine (a) the isentropic efficiency and (b) the second-law efficiency of the compressor.
FIGURE P8–61
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Steam enters an adiabatic turbine at 45 MPa and 840°C with a mass flow rate of 69kg/s and leaves at 30 kPa. The isentropic efficiency of the turbine is 0.90. Neglecting thekinetic energy change of the steam, determine (a) the temperature at the turbine exit and(b) the power output of the turbine.
Air is compressed in an adiabatic compressor from 55 psia and 660 R to 270 psia and 1200 R. Calculate the isentropic efficiency of the compressor. Type your answer in the first box.
Refrigerant-134a at 140 kPa and –10°C is compressed by an adiabatic 0.5-kW compressor to an exit state of 700 kPa and 60°C. Neglecting the changes in kinetic and potential energies and assuming the surroundings to be at 27°C, determine the second-law efficiency of the compressor.
Chapter 8 Solutions
Thermodynamics: An Engineering Approach
Ch. 8.8 - What final state will maximize the work output of...Ch. 8.8 - Is the exergy of a system different in different...Ch. 8.8 - Under what conditions does the reversible work...Ch. 8.8 - How does useful work differ from actual work? For...Ch. 8.8 - How does reversible work differ from useful work?Ch. 8.8 - Is a process during which no entropy is generated...Ch. 8.8 - Consider an environment of zero absolute pressure...Ch. 8.8 - It is well known that the actual work between the...Ch. 8.8 - Consider two geothermal wells whose energy...Ch. 8.8 - Consider two systems that are at the same pressure...
Ch. 8.8 - Prob. 11PCh. 8.8 - Does a power plant that has a higher thermal...Ch. 8.8 - Prob. 13PCh. 8.8 - Saturated steam is generated in a boiler by...Ch. 8.8 - One method of meeting the extra electric power...Ch. 8.8 - A heat engine that receives heat from a furnace at...Ch. 8.8 - Consider a thermal energy reservoir at 1500 K that...Ch. 8.8 - A heat engine receives heat from a source at 1100...Ch. 8.8 - A heat engine that rejects waste heat to a sink at...Ch. 8.8 - A geothermal power plant uses geothermal liquid...Ch. 8.8 - A house that is losing heat at a rate of 35,000...Ch. 8.8 - A freezer is maintained at 20F by removing heat...Ch. 8.8 - Prob. 24PCh. 8.8 - Prob. 25PCh. 8.8 - Prob. 26PCh. 8.8 - Can a system have a higher second-law efficiency...Ch. 8.8 - A mass of 8 kg of helium undergoes a process from...Ch. 8.8 - Which is a more valuable resource for work...Ch. 8.8 - Which has the capability to produce the most work...Ch. 8.8 - The radiator of a steam heating system has a...Ch. 8.8 - A well-insulated rigid tank contains 6 lbm of a...Ch. 8.8 - A pistoncylinder device contains 8 kg of...Ch. 8.8 - Prob. 35PCh. 8.8 - Prob. 36PCh. 8.8 - Prob. 37PCh. 8.8 - A pistoncylinder device initially contains 2 L of...Ch. 8.8 - A 0.8-m3 insulated rigid tank contains 1.54 kg of...Ch. 8.8 - An insulated pistoncylinder device initially...Ch. 8.8 - Prob. 41PCh. 8.8 - An insulated rigid tank is divided into two equal...Ch. 8.8 - A 50-kg iron block and a 20-kg copper block, both...Ch. 8.8 - Prob. 45PCh. 8.8 - Prob. 46PCh. 8.8 - Prob. 47PCh. 8.8 - A pistoncylinder device initially contains 1.4 kg...Ch. 8.8 - Prob. 49PCh. 8.8 - Prob. 50PCh. 8.8 - Prob. 51PCh. 8.8 - Air enters a nozzle steadily at 200 kPa and 65C...Ch. 8.8 - Prob. 54PCh. 8.8 - Prob. 55PCh. 8.8 - Argon gas enters an adiabatic compressor at 120...Ch. 8.8 - Prob. 57PCh. 8.8 - Prob. 58PCh. 8.8 - The adiabatic compressor of a refrigeration system...Ch. 8.8 - Refrigerant-134a at 140 kPa and 10C is compressed...Ch. 8.8 - Air enters a compressor at ambient conditions of...Ch. 8.8 - Combustion gases enter a gas turbine at 900C, 800...Ch. 8.8 - Steam enters a turbine at 9 MPa, 600C, and 60 m/s...Ch. 8.8 - Refrigerant-134a is condensed in a refrigeration...Ch. 8.8 - Prob. 66PCh. 8.8 - Refrigerant-22 absorbs heat from a cooled space at...Ch. 8.8 - Prob. 68PCh. 8.8 - Prob. 69PCh. 8.8 - Air enters a compressor at ambient conditions of...Ch. 8.8 - Hot combustion gases enter the nozzle of a...Ch. 8.8 - Prob. 72PCh. 8.8 - A 0.6-m3 rigid tank is filled with saturated...Ch. 8.8 - Prob. 74PCh. 8.8 - Prob. 75PCh. 8.8 - An insulated vertical pistoncylinder device...Ch. 8.8 - Liquid water at 200 kPa and 15C is heated in a...Ch. 8.8 - Prob. 78PCh. 8.8 - Prob. 79PCh. 8.8 - A well-insulated shell-and-tube heat exchanger is...Ch. 8.8 - Steam is to be condensed on the shell side of a...Ch. 8.8 - Prob. 82PCh. 8.8 - Prob. 83PCh. 8.8 - Prob. 84PCh. 8.8 - Prob. 85RPCh. 8.8 - Prob. 86RPCh. 8.8 - An aluminum pan has a flat bottom whose diameter...Ch. 8.8 - Prob. 88RPCh. 8.8 - Prob. 89RPCh. 8.8 - A well-insulated, thin-walled, counterflow heat...Ch. 8.8 - Prob. 92RPCh. 8.8 - Prob. 93RPCh. 8.8 - Prob. 94RPCh. 8.8 - Prob. 95RPCh. 8.8 - Nitrogen gas enters a diffuser at 100 kPa and 110C...Ch. 8.8 - Prob. 97RPCh. 8.8 - Steam enters an adiabatic nozzle at 3.5 MPa and...Ch. 8.8 - Prob. 99RPCh. 8.8 - A pistoncylinder device initially contains 8 ft3...Ch. 8.8 - An adiabatic turbine operates with air entering at...Ch. 8.8 - Steam at 7 MPa and 400C enters a two-stage...Ch. 8.8 - Prob. 103RPCh. 8.8 - Steam enters a two-stage adiabatic turbine at 8...Ch. 8.8 - Prob. 105RPCh. 8.8 - Prob. 106RPCh. 8.8 - Prob. 107RPCh. 8.8 - Prob. 108RPCh. 8.8 - Prob. 109RPCh. 8.8 - Prob. 111RPCh. 8.8 - A passive solar house that was losing heat to the...Ch. 8.8 - Prob. 113RPCh. 8.8 - A 4-L pressure cooker has an operating pressure of...Ch. 8.8 - Repeat Prob. 8114 if heat were supplied to the...Ch. 8.8 - Prob. 116RPCh. 8.8 - A rigid 50-L nitrogen cylinder is equipped with a...Ch. 8.8 - Prob. 118RPCh. 8.8 - Prob. 119RPCh. 8.8 - Prob. 120RPCh. 8.8 - Reconsider Prob. 8-120. The air stored in the tank...Ch. 8.8 - Prob. 122RPCh. 8.8 - Prob. 123RPCh. 8.8 - Prob. 124RPCh. 8.8 - Prob. 125RPCh. 8.8 - Prob. 126RPCh. 8.8 - Prob. 127RPCh. 8.8 - Water enters a pump at 100 kPa and 30C at a rate...Ch. 8.8 - Prob. 129RPCh. 8.8 - Prob. 130RPCh. 8.8 - Obtain a relation for the second-law efficiency of...Ch. 8.8 - Writing the first- and second-law relations and...Ch. 8.8 - Prob. 133RPCh. 8.8 - Keeping the limitations imposed by the second law...Ch. 8.8 - Prob. 135FEPCh. 8.8 - Prob. 136FEPCh. 8.8 - Prob. 137FEPCh. 8.8 - Prob. 138FEPCh. 8.8 - A furnace can supply heat steadily at 1300 K at a...Ch. 8.8 - A heat engine receives heat from a source at 1500...Ch. 8.8 - Air is throttled from 50C and 800 kPa to a...Ch. 8.8 - Prob. 142FEPCh. 8.8 - A 12-kg solid whose specific heat is 2.8 kJ/kgC is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- The exhaust nozzle of a jet engine expands air at 300 kPa and 180°C adiabatically to 100 kPa. Determine the air velocity at the exit when the inlet velocity is low and the nozzle isentropic efficiency is 93 percent.arrow_forwardRefrigerant-134a at 140 kPa and –10°C is compressed by an adiabatic 1.3-kW compressor to an exit state of 700 kPa and 60°C. Neglecting the changes in kinetic and potential energies, determine the isentropic efficiency of the compressor.arrow_forwardSaturated refrigerant-134a vapor at 15 psia is compressed reversibly in an adiabatic compressor to 80 psia. Determine the work input to the compressor. What would your answer be if the refrigerant were first condensed at constant pressure before it was compressed?arrow_forward
- An adiabatic steam nozzle has steam entering at 500 kPa, 200°C, and 30 m/s, and leaving as a saturated vapor at 200 kPa. Calculate the second-law efficiency of the nozzle. Take T0 = 25°Carrow_forwardRefrigerant-134a is expanded adiabatically from 100 psia and 100°F to a pressure of 10 psia. Determine the entropy generation for this process, in Btu/lbm·R.arrow_forwardAir is compressed by an adiabatic compressor from 95 kPa and 27°C to 600 kPa and 277°C. Assuming variable specific heats and neglecting the changes in kinetic and potential energies, determine the isentropic efficiency of the compressor. List your assumptions.arrow_forward
- Air enters the evaporator section of a window air conditioner at 100 kPa and 27°C with a volume flow rate of 6 m3 /min. The refrigerant-134a at 120 kPa with a quality of 0.3 enters the evaporator at a rate of 2 kg/min and leaves as saturated vapor at the same pressure. Determine the exit temperature of the air and the rate of entropy generation for this process, assuming heat is transferred to the evaporator of the air conditioner from the surrounding medium at 32°C at a rate of 30 kJ/min.arrow_forwardSteam enters an adiabatic nozzle at 2 MPa and 350°C with a velocity of 55 m/s and exits at 0.8 MPa and 390 m/s. If the nozzle has an inlet area of 7.5 cm2 , determine the rate of entropy generation for this process.arrow_forwardAir is compressed by an adiabatic compressor from 95 kPa and 27°C to 600 kPa and 277°C. Assuming variable specific heats and neglecting the changes in kinetic and potential energies, determine the exit temperature of air if the process were reversible.arrow_forward
- Steam enters an adiabatic turbine steadily at 7 MPa, 500°C, and 45 m/s and leaves at 100 kPa and 75 m/s. If the power output of the turbine is 5 MW and the isentropic efficiency is 77 percent, determine the temperature at the turbine exit.arrow_forwardSteam enters an adiabatic turbine at P1 MPa and T1°C with a mass flow rate of z kg/s and leaves at 30 kPa. The isentropic efficiency of the turbine is 0.90. Neglecting the kinetic energy change of the steam, determine (a) the temperature at the turbine exit and(b) the power output of the turbine.arrow_forwardA 100-lbm block of a solid material whose specific heat is 0.5 Btu/lbm·R is at 80°F. It is heated with 10 lbm of saturated water vapor that has a constant pressure of 20 psia. Determine the final temperature of the block and water, and the entropy change of the block.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Intro to Compressible Flows — Lesson 1; Author: Ansys Learning;https://www.youtube.com/watch?v=OgR6j8TzA5Y;License: Standard Youtube License