
Thermodynamics: An Engineering Approach
9th Edition
ISBN: 9781260048766
Author: CENGEL
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 8.8, Problem 104RP
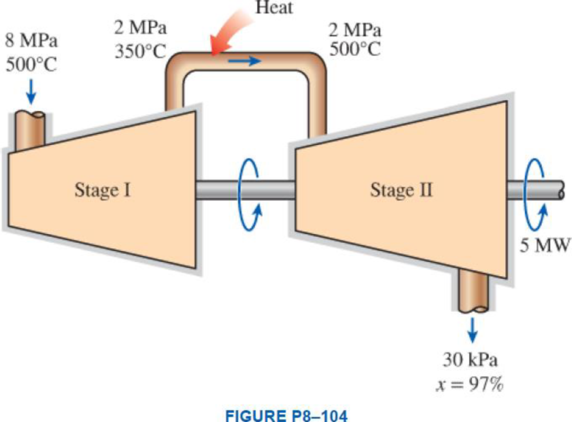
Steam enters a two-stage adiabatic turbine at 8 MPa and 500°C. It expands in the first stage to a state of 2 MPa and 350°C. Steam is then reheated at constant pressure to a temperature of 500°C before it is routed to the second stage, where it exits at 30 kPa and a quality of 97 percent. The work output of the turbine is 5 MW. Assuming the surroundings to be at 25°C, determine the reversible power output and the rate of exergy destruction within this turbine.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A rigid, insulated tank that is initially evacuated is connected through a valve to a supply line that carries steam at 4 MPa. Now the valve is opened, and steam is allowed to flow into the tank until the pressure reaches 4 MPa, at which point the valve is closed. If the final temperature of the steam in the tank is 550°C, determine the temperature of the steam in the supply line and the flow work per unit mass of the steam.
Steam enters the condenser of a steam power plant at 20000 kPa and a quality of 95
percent with a mass flow rate of 20 Mg/h. It is to be cooled by water from a nearby river
in circulating the water through the tubes within the condenser. To prevent thermal
pollution, the river water is not allowed to experience a temperature rise above 10°C. If
the steam is to leave the condenser as saturated liquid at 20000 Pa, determine the mass
flow rate of the cooling water required.
m = 20,000 kg/h
P = 20 kPa
= 0.95
%3D
Steam
Water
T+ 10°C
P = 20 kPa
Sat. liquid
Thermodynamics
Chapter 8 Solutions
Thermodynamics: An Engineering Approach
Ch. 8.8 - What final state will maximize the work output of...Ch. 8.8 - Is the exergy of a system different in different...Ch. 8.8 - Under what conditions does the reversible work...Ch. 8.8 - How does useful work differ from actual work? For...Ch. 8.8 - How does reversible work differ from useful work?Ch. 8.8 - Is a process during which no entropy is generated...Ch. 8.8 - Consider an environment of zero absolute pressure...Ch. 8.8 - It is well known that the actual work between the...Ch. 8.8 - Consider two geothermal wells whose energy...Ch. 8.8 - Consider two systems that are at the same pressure...
Ch. 8.8 - Prob. 11PCh. 8.8 - Does a power plant that has a higher thermal...Ch. 8.8 - Prob. 13PCh. 8.8 - Saturated steam is generated in a boiler by...Ch. 8.8 - One method of meeting the extra electric power...Ch. 8.8 - A heat engine that receives heat from a furnace at...Ch. 8.8 - Consider a thermal energy reservoir at 1500 K that...Ch. 8.8 - A heat engine receives heat from a source at 1100...Ch. 8.8 - A heat engine that rejects waste heat to a sink at...Ch. 8.8 - A geothermal power plant uses geothermal liquid...Ch. 8.8 - A house that is losing heat at a rate of 35,000...Ch. 8.8 - A freezer is maintained at 20F by removing heat...Ch. 8.8 - Prob. 24PCh. 8.8 - Prob. 25PCh. 8.8 - Prob. 26PCh. 8.8 - Can a system have a higher second-law efficiency...Ch. 8.8 - A mass of 8 kg of helium undergoes a process from...Ch. 8.8 - Which is a more valuable resource for work...Ch. 8.8 - Which has the capability to produce the most work...Ch. 8.8 - The radiator of a steam heating system has a...Ch. 8.8 - A well-insulated rigid tank contains 6 lbm of a...Ch. 8.8 - A pistoncylinder device contains 8 kg of...Ch. 8.8 - Prob. 35PCh. 8.8 - Prob. 36PCh. 8.8 - Prob. 37PCh. 8.8 - A pistoncylinder device initially contains 2 L of...Ch. 8.8 - A 0.8-m3 insulated rigid tank contains 1.54 kg of...Ch. 8.8 - An insulated pistoncylinder device initially...Ch. 8.8 - Prob. 41PCh. 8.8 - An insulated rigid tank is divided into two equal...Ch. 8.8 - A 50-kg iron block and a 20-kg copper block, both...Ch. 8.8 - Prob. 45PCh. 8.8 - Prob. 46PCh. 8.8 - Prob. 47PCh. 8.8 - A pistoncylinder device initially contains 1.4 kg...Ch. 8.8 - Prob. 49PCh. 8.8 - Prob. 50PCh. 8.8 - Prob. 51PCh. 8.8 - Air enters a nozzle steadily at 200 kPa and 65C...Ch. 8.8 - Prob. 54PCh. 8.8 - Prob. 55PCh. 8.8 - Argon gas enters an adiabatic compressor at 120...Ch. 8.8 - Prob. 57PCh. 8.8 - Prob. 58PCh. 8.8 - The adiabatic compressor of a refrigeration system...Ch. 8.8 - Refrigerant-134a at 140 kPa and 10C is compressed...Ch. 8.8 - Air enters a compressor at ambient conditions of...Ch. 8.8 - Combustion gases enter a gas turbine at 900C, 800...Ch. 8.8 - Steam enters a turbine at 9 MPa, 600C, and 60 m/s...Ch. 8.8 - Refrigerant-134a is condensed in a refrigeration...Ch. 8.8 - Prob. 66PCh. 8.8 - Refrigerant-22 absorbs heat from a cooled space at...Ch. 8.8 - Prob. 68PCh. 8.8 - Prob. 69PCh. 8.8 - Air enters a compressor at ambient conditions of...Ch. 8.8 - Hot combustion gases enter the nozzle of a...Ch. 8.8 - Prob. 72PCh. 8.8 - A 0.6-m3 rigid tank is filled with saturated...Ch. 8.8 - Prob. 74PCh. 8.8 - Prob. 75PCh. 8.8 - An insulated vertical pistoncylinder device...Ch. 8.8 - Liquid water at 200 kPa and 15C is heated in a...Ch. 8.8 - Prob. 78PCh. 8.8 - Prob. 79PCh. 8.8 - A well-insulated shell-and-tube heat exchanger is...Ch. 8.8 - Steam is to be condensed on the shell side of a...Ch. 8.8 - Prob. 82PCh. 8.8 - Prob. 83PCh. 8.8 - Prob. 84PCh. 8.8 - Prob. 85RPCh. 8.8 - Prob. 86RPCh. 8.8 - An aluminum pan has a flat bottom whose diameter...Ch. 8.8 - Prob. 88RPCh. 8.8 - Prob. 89RPCh. 8.8 - A well-insulated, thin-walled, counterflow heat...Ch. 8.8 - Prob. 92RPCh. 8.8 - Prob. 93RPCh. 8.8 - Prob. 94RPCh. 8.8 - Prob. 95RPCh. 8.8 - Nitrogen gas enters a diffuser at 100 kPa and 110C...Ch. 8.8 - Prob. 97RPCh. 8.8 - Steam enters an adiabatic nozzle at 3.5 MPa and...Ch. 8.8 - Prob. 99RPCh. 8.8 - A pistoncylinder device initially contains 8 ft3...Ch. 8.8 - An adiabatic turbine operates with air entering at...Ch. 8.8 - Steam at 7 MPa and 400C enters a two-stage...Ch. 8.8 - Prob. 103RPCh. 8.8 - Steam enters a two-stage adiabatic turbine at 8...Ch. 8.8 - Prob. 105RPCh. 8.8 - Prob. 106RPCh. 8.8 - Prob. 107RPCh. 8.8 - Prob. 108RPCh. 8.8 - Prob. 109RPCh. 8.8 - Prob. 111RPCh. 8.8 - A passive solar house that was losing heat to the...Ch. 8.8 - Prob. 113RPCh. 8.8 - A 4-L pressure cooker has an operating pressure of...Ch. 8.8 - Repeat Prob. 8114 if heat were supplied to the...Ch. 8.8 - Prob. 116RPCh. 8.8 - A rigid 50-L nitrogen cylinder is equipped with a...Ch. 8.8 - Prob. 118RPCh. 8.8 - Prob. 119RPCh. 8.8 - Prob. 120RPCh. 8.8 - Reconsider Prob. 8-120. The air stored in the tank...Ch. 8.8 - Prob. 122RPCh. 8.8 - Prob. 123RPCh. 8.8 - Prob. 124RPCh. 8.8 - Prob. 125RPCh. 8.8 - Prob. 126RPCh. 8.8 - Prob. 127RPCh. 8.8 - Water enters a pump at 100 kPa and 30C at a rate...Ch. 8.8 - Prob. 129RPCh. 8.8 - Prob. 130RPCh. 8.8 - Obtain a relation for the second-law efficiency of...Ch. 8.8 - Writing the first- and second-law relations and...Ch. 8.8 - Prob. 133RPCh. 8.8 - Keeping the limitations imposed by the second law...Ch. 8.8 - Prob. 135FEPCh. 8.8 - Prob. 136FEPCh. 8.8 - Prob. 137FEPCh. 8.8 - Prob. 138FEPCh. 8.8 - A furnace can supply heat steadily at 1300 K at a...Ch. 8.8 - A heat engine receives heat from a source at 1500...Ch. 8.8 - Air is throttled from 50C and 800 kPa to a...Ch. 8.8 - Prob. 142FEPCh. 8.8 - A 12-kg solid whose specific heat is 2.8 kJ/kgC is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A rigid tank initially contains 0.9 kg of water. Then it is filled more with steam from a supply line at 1.5 MPa and 320°C until the pressure in the tank reaches to the supply pressure (1.5 MPa) at 360°C. The final mass in the tank is 2 kg and the process is adiabatic. • Calculate the initial specific internal energy of the water. • Comment on the initial phase of the water. Is it mixture or superheated or?. steam supply line initially 0.9 kg waterarrow_forwardThe compressors of a production facility maintain the compressed-air lines at a (gauge) pressure of 700 kPa at sea level where the atmospheric pressure is 101 kPa. The average temperature of air is 20°C at the compressor inlet and 24°C in the compressed-air lines. The facility operates 4200 hours a year, and the average price of electricity is $0.078/kWh. Taking the compressor efficiency to be 0.8, the motor efficiency to be 0.92, and the discharge coefficient to be 0.65, determine the energy and money saved per year by sealing a leak equivalent to a 3-mm-diameter hole on the compressed-air line.arrow_forward. A piston–cylinder device contains steam that undergoes a reversible thermodynamic cycle.Initially the steam is at 400 kPa and 350°C with a volume of 0.5 m3. The steam is first expandedisothermally to 150 kPa, then compressed adiabatically to the initial pressure, and finallycompressed at the constant pressure to the initial state. Determine the net work and heattransfer for the cycle after you calculate the work and heat interaction for each process.arrow_forward
- Steam at 6000 kPa and 500°C enters a steady-flow turbine. The steam expands in the turbine while doing work until the pressure is 1000 kPa. When the pressure is 1000 kPa, 10 percent of the steam is removed from the turbine for other uses. The remaining 90 percent of the steam continues to expand through the turbine while doing work and leaves the turbine at 10 kPa. The entire expansion process by the steam through the turbine is reversible and adiabatic. Sketch the process on a T-s diagram with respect to the saturation lines. Be sure to label the data states and the lines of constant pressure?arrow_forwardSteam at 6000 kPa and 500°C enters a steady-flow turbine. The steam expands in the turbine while doing work until the pressure is 1000 kPa. When the pressure is 1000 kPa, 10 percent of the steam is removed from the turbine for other uses. The remaining 90 percent of the steam continues to expand through the turbine while doing work and leaves the turbine at 10 kPa. The entire expansion process by the steam through the turbine is reversible and adiabatic. If the turbine has an isentropic efficiency of 85 percent, what is the work done by the steam as it flows through the turbine per unit mass of steam flowing into the turbine, in kJ/kg?arrow_forwardAir at 27°C and 100 kPa is contained in a piston– cylinder device. When the air is compressed adiabatically, a minimum work input of 1000 kJ will increase the pressure to 600 kPa. Assuming air has constant specific heats evaluated at 300 K, determine the mass of air in the device.arrow_forward
- Steam enters the condenser of a steam power plant at 50 kPa and a quality of 85 percent with a mass flow rate of 400 kg/min. It is to be cooled by water from a nearby river by circulating the water through the tubes within the condenser. To prevent thermal pollution, the river water is not allowed to experience a temperature rise above 20°C. If the steam is to leave the condenser as saturated liquid at 50 kPa, determine the mass flow rate of the cooling water required.arrow_forwardAir at 17 oC is compressed steadily in an adiabatic compressor from 100 kPa to 680 kPa. If the minimum power consumption of this compressor is 7 kW, determine the mass flow rate to run the compressor considering the variation of specific heats with temperature. Note: Do NOT perform interpolation, take the closest value.arrow_forwardAn open feedwater heater heats the feedwater by mixing it with hot steam. Consider an electric power plant with an open feedwater heater that mixes 0.1 lbm/s of steam at 10 psia and 200°F with 2.0 lbm/s of feedwater at 10 psia and 100°F to produce 10 psia and 120°F feedwater at the outlet. The diameter of the outlet pipe is 0.54 ft. Determine the mass flow rate and feedwater velocity at the outlet. Use data from the steam tables.arrow_forward
- An open feedwater heater heats the feedwater by mixing it with hot steam. Consider an electric power plant with an open feedwater heater that mixes 0.1 lbm/s of steam at 10 psia and 200°F with 2.0 lbm/s of feedwater at 10 psia and 100°F to produce 10 psia and 120°F feedwater at the outlet. The diameter of the outlet pipe is 0.5 ft. Determine the mass flow rate and feedwater velocity at the outlet. Would the outlet flow rate and velocity be significantly different if the temperature at the outlet were 180°F?arrow_forwardRefrigerant-134a is compressed steadily from the saturated vapor state at 120 kPa to 1.6 MPa and 60 °C at a rate of 0.27 kg/s. The compressor is losing heat at a rate of 1.6 kJ/s during compression. Determine the power input to the compressor. O10.0 kW 11.8 kW O 13.4 kW O 21.0 kWarrow_forwardAn air compressor takes in air at a pressure of 100 kPa and a temperature of 300 K. The compressor is cooled at a rate of 15 k/kg and the mass flow rate is 50 kg/minute. The air leaves at a pressure of 600 kPa and a temperature of 420 K. What is the work that needs to be input to the compressor to achieve this rate of flow? You may use a cp of 1.006 k/kg-K. Give your answer to three significant digits in kilowatts.don't put kw in your answerl) COMPRESSOR SECTION Compressor housing Compressor a dicharge Compreor anbient a Compreor wheelarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Power Plant Explained | Working Principles; Author: RealPars;https://www.youtube.com/watch?v=HGVDu1z5YQ8;License: Standard YouTube License, CC-BY