
(a)
Interpretation:
The element with its full and condensed electron configuration and the number of inner electrons is to be determined from the given partial orbital diagram.
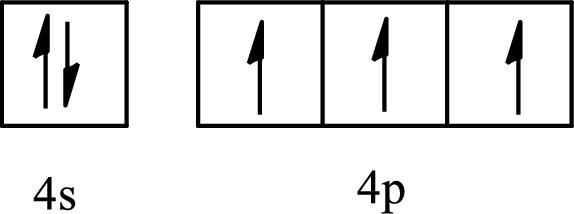
Concept introduction:
The electronic configuration tells about the distribution of electrons in various atomic orbitals.
The full electronic configuration of an atom tells about the distribution of electrons in its various atomic orbital.
The condensed electronic configuration is a way to write the electronic configuration where the inner shell configurations are compressed to the nearest noble gas configuration and only the valence shell configuration is written in the expanded form.
The partial orbital diagram is the one that shows the distribution of valence electrons only.
The inner electrons are present in the inner orbitals of the atom and the valence electrons are present in the outermost shell of the atom.
The number of inner electrons is calculated by subtracting the valence electrons from the total number of electrons
(b)
Interpretation:
The element with its full and condensed electron configuration and the number of inner electrons is to be determined from the given partial orbital diagram.
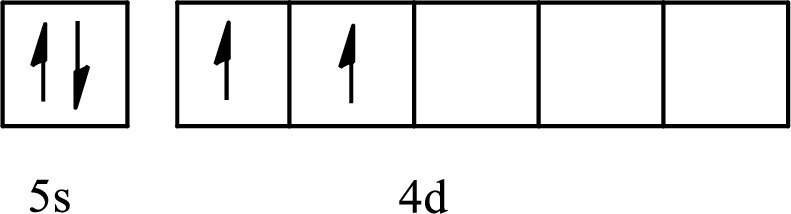
Concept introduction:
The electronic configuration tells about the distribution of electrons in various atomic orbitals.
The full electronic configuration of an atom tells about the distribution of electrons in its various atomic orbital.
The condensed electronic configuration is a way to write the electronic configuration where the inner shell configurations are compressed to the nearest noble gas configuration and only the valence shell configuration is written in the expanded form.
The partial orbital diagram is the one that shows the distribution of valence electrons only.
The inner electrons are present in the inner orbitals of the atom and the valence electrons are present in the outermost shell of the atom.
The number of inner electrons is calculated by subtracting the valence electrons from the total number of electrons
(c)
Interpretation:
The element with its full and condensed electron configuration and the number of inner electrons is to be determined from the given partial orbital diagram.
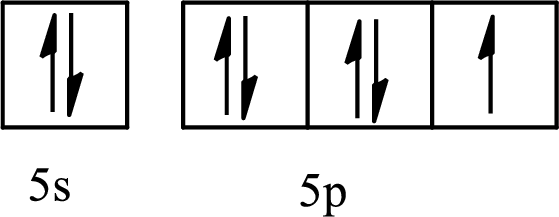
Concept introduction:
The electronic configuration tells about the distribution of electrons in various atomic orbitals.
The full electronic configuration of an atom tells about the distribution of electrons in its various atomic orbital.
The condensed electronic configuration is a way to write the electronic configuration where the inner shell configurations are compressed to the nearest noble gas configuration and only the valence shell configuration is written in the expanded form.
The partial orbital diagram is the one that shows the distribution of valence electrons only.
The inner electrons are present in the inner orbitals of the atom and the valence electrons are present in the outermost shell of the atom.
The number of inner electrons is calculated by subtracting the valence electrons from the total number of electrons
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
CHEMISTRY/ALEKS AND CONNECT
- Q8. Draw the mechanism for this halogenation reaction. Show all steps including initiation, propagation, and recombination. Cl₂, hv CI Br Br2, hv, heatarrow_forwardQ6. Given the following alkanes, draw the most likely product to form upon monohalogenation with Br2 (keep in mind that this may not be the only product to form though). If the reaction was performed with Cl2 would there be more or less selectivity in the desired product formation? Why? (a) (b) (c)arrow_forwardQ4. Radicals a. For the following indicated bonds, rank them in order of decreasing AH° for homolytic cleavage. Based on your answer, which bond would be most likely to break homolytically? (c) CH3 CH3 H3C CH3 (a) CH3 (b)arrow_forward
- Q1. (a) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH3. Use curved arrows to show the electron movement. (b) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH4*. Use curved arrows to show the electron movement.arrow_forwardohing Quantitative Relationships 425 The specific heats and atomic masses of 20 of the elements are given in the table below. Use a graphical method to determine if there is a relationship between specific heat and the atomic mass. a. b. C. d. e. If your graphs revealed relationship between specific heat and atomic revealed a mathematical mass, write down an equation for the relationship. Comment on the usefulness of the determination of specific heat as a method for identifying an element. Would specific heat alone give you much confidence with regard to the identity of the element? If you think measurement of another property would be needed to support an identification, what property would you measure and why? The elements listed in the table are all selected metals. The values for nitrogen, oxygen, fluorine and neon are 1.040, 0.918, 0.824 and 1.030 J/g K respectively. Do these elements fit your equation? element atomic mass specific heat (almol) (Jig K) magnesium 24.305 1.023…arrow_forwardPlease correct answer and don't use hand rating and don't use Ai solutionarrow_forward
- Nonearrow_forwardDraw Newman projects for each of the following molecules with 3 different rotational angles from carbon 2 to carbon 3. Rank your structures from lowest to highest energy. What causes the energy differences? Label the overlap. a. b. Br OH C. Br Brarrow_forwardDraw the stereoisomers of 3,5-diethylcylopentane. Identify the different relationships between each molecules (diasteromers, enantiomers, meso compounds, etc.)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





