
Interpretation:
For the given reaction, whether an efficient Michael reaction is expected or not has to be determined.
Concept Introduction:
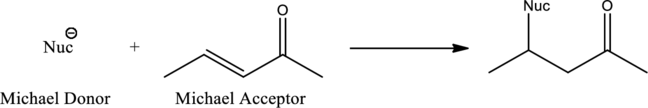
Michael reaction is the reaction between a Michael donor which acts as a nucleophile and a Michael acceptor. Important condition for the Michael reaction to occur is the formation of stabilized nucleophile. 1,4-addition is known as Michael addition where the beta-keto compound acts as nucleophile. General scheme for Michael reaction can be given as,
Explanation of Solution
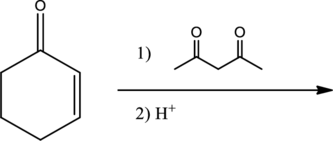
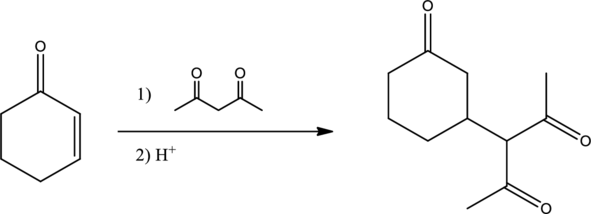
Given reaction is,
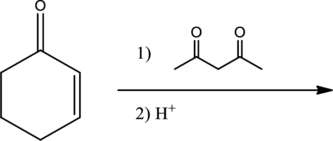
From the above reaction, it is found that there is an alpha,beta-unsaturated
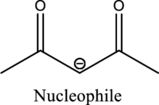
Therefore, the given reaction will be an efficient Michael reaction. It can be represented as shown below,
Want to see more full solutions like this?
Chapter 8 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
- I need the nomenclature of this compoundarrow_forward2. Name the following hydrocarbons. (9 marks) a) HHHHHHHH H-C-C- H-O-S b) HCEC-CH3 H H H H H d) c) H C=C- H H H e) CH3 CH3 CH2CH=CH-CH=CHCH3 HHHH H-C-C-C-C-H H HH H f) large CH2CH3 pola H3C section lovels tower, able ocart firs g) Tower H3C-CH2 then in H3C-CH-CH-CH3 enblbano bne noitsidab Copyright © 2008. Durham Continuing Education CH3arrow_forwardName the molecules & Identify any chiral center CH3CH2CH2CHCH₂CH₂CH₂CH₂ OH CH₂CHCH2CH3 Br CH3 CH3CHCH2CHCH2CH3 CH3arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
