
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 8.9, Problem 8.69P
Interpretation Introduction
Interpretation:
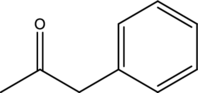
Reason for the given compound cannot be prepared by acetoacetic ester synthesis has to be given.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Absorbance and transmittance are related by:
A = -log(T)
A solution has a transmittance of 35% in a 1-cm-pathlength cell at a certain wavelength. Calculate the transmittance if you dilute 25.0 mL of the solution to 50.0 mL? (A = εbc)
What is the transmittance of the original solution if the pathlength is increased to 10 cm?
Under what conditions will Beer’s Law most likely NO LONGER be linear?
When the absorbing species is very dilute.
When the absorbing species participates in a concentration-dependent equilibrium.
When the solution being studied contains a mixture of ions.
Compared to incident (exciting) radiation, fluorescence emission will have a:
Higher energy
Higher frequency
Longer wavelength
Chapter 8 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 8.1 - For each of the compounds below, identify all...Ch. 8.1 - For each of the compounds below, identify all...Ch. 8.1 - For each of the compounds below, identify all...Ch. 8.1 - For each of the compounds below, identify all...Ch. 8.1 - For each of the compounds below, identify all...Ch. 8.1 - For each of the compounds below, identify all...Ch. 8.2 - Prob. 8.9PCh. 8.2 - Prob. 8.10PCh. 8.2 - Prob. 8.11PCh. 8.2 - Prob. 8.12P
Ch. 8.2 - Prob. 8.13PCh. 8.3 - Predict the products of each of the following...Ch. 8.3 - Predict the products of each of the following...Ch. 8.3 - Predict the products of each of the following...Ch. 8.3 - Predict the products of each of the following...Ch. 8.4 - Prob. 8.20PCh. 8.4 - Prob. 8.21PCh. 8.4 - Prob. 8.22PCh. 8.4 - Prob. 8.23PCh. 8.5 - Prob. 8.25PCh. 8.5 - Prob. 8.26PCh. 8.5 - On a separate piece of paper, draw a mechanism for...Ch. 8.6 - Prob. 8.29PCh. 8.6 - Predict the major product for each of the...Ch. 8.6 - Predict the major product for each of the...Ch. 8.6 - Predict the major product for each of the...Ch. 8.6 - Identify the reagents you would use to achieve...Ch. 8.6 - Identify the reagents you would use to achieve...Ch. 8.6 - Identify the reagents you would use to achieve...Ch. 8.7 - Predict the major product for each of the...Ch. 8.7 - Predict the major product for each of the...Ch. 8.7 - Predict the major product for each of the...Ch. 8.7 - Prob. 8.42PCh. 8.7 - Prob. 8.43PCh. 8.7 - Prob. 8.44PCh. 8.7 - Prob. 8.45PCh. 8.7 - Prob. 8.47PCh. 8.7 - Prob. 8.48PCh. 8.7 - Prob. 8.49PCh. 8.7 - Prob. 8.50PCh. 8.8 - Prob. 8.52PCh. 8.8 - Prob. 8.53PCh. 8.8 - Prob. 8.54PCh. 8.8 - Prob. 8.55PCh. 8.8 - Prob. 8.57PCh. 8.8 - Prob. 8.58PCh. 8.8 - Prob. 8.59PCh. 8.8 - Prob. 8.60PCh. 8.8 - Propose a mechanism for each of the following...Ch. 8.8 - Propose a mechanism for each of the following...Ch. 8.8 - Prob. 8.64PCh. 8.9 - Prob. 8.66PCh. 8.9 - Prob. 8.67PCh. 8.9 - Prob. 8.68PCh. 8.9 - Prob. 8.69PCh. 8.9 - Prob. 8.70PCh. 8.9 - Prob. 8.71PCh. 8.9 - Prob. 8.72PCh. 8.9 - Identify what reagents you would use to achieve...Ch. 8.9 - Identify what reagents you would use to achieve...Ch. 8.9 - Identify what reagents you would use to achieve...Ch. 8.10 - Prob. 8.78PCh. 8.10 - Prob. 8.79PCh. 8.10 - Prob. 8.80PCh. 8.10 - Propose a synthesis for each of the following...Ch. 8.10 - Propose a synthesis for each of the following...Ch. 8.10 - Propose a synthesis for each of the following...Ch. 8.10 - Propose a synthesis for each of the following...
Knowledge Booster
Similar questions
- Lin and Brown described a quantitative method for methanol based on its effect on the visible spectrum of methylene blue. In the absence of methanol, methylene blue has two prominent absorption bands at 610 nm and 663 nm, which correspond to the monomer and the dimer, respectively. In the presence of methanol, the intensity of the dimer’s absorption band decreases, while that for the monomer increases. For concentrations of methanol between 0 and 30% v/v, the ratio of the two absorbance, A663/ A610, is a linear function of the amount of methanol. Use the following standardization data to determine the %v/v methanol in a sample if A610 is 0.75 and A663 is 1.07.arrow_forwardThe crystal field splitting energy, Δ, of a complex is determined to be 2.9 × 10-19 What wavelength of light would this complex absorb? What color of light is this? What color would the compound be in solution?arrow_forwardA key component of a monochromator is the exit slit. As the exit slit is narrowed, the bandwidth of light (i.e., the range of wavelengths) exiting the slit gets smaller, leading to higher resolution. What is a possible disadvantage of narrowing the exit slit? (Hint: why might a narrower slit lower the sensitivity of the measurement?).arrow_forward
- An x-ray has a frequency of 3.33 × 1018 What is the wavelength of this light?arrow_forwardChoose the Lewis structure for the compound below: H2CCHOCH2CH(CH3)2 HH H :d H H H C. Η H H HH H H H H. H H H HH H H H H H- H H H C-H H H HHHHarrow_forwardEach of the highlighted carbon atoms is connected to hydrogen atoms.arrow_forward
- く Complete the reaction in the drawing area below by adding the major products to the right-hand side. If there won't be any products, because nothing will happen under these reaction conditions, check the box under the drawing area instead. Note: if the products contain one or more pairs of enantiomers, don't worry about drawing each enantiomer with dash and wedge bonds. Just draw one molecule to represent each pair of enantiomers, using line bonds at the chiral center. More... No reaction. Explanation Check O + G 1. Na O Me Click and drag to start drawing a structure. 2. H + 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 000 Ar Parrow_forwardDraw a tetramer of this alternating copolymer.arrow_forwardH I T H HH H -H C. H- Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H CIH H H H HHHH H H 0 ·H H– 冊 CH CHI HH C- H- H H- H H A. H H C H H- -H HH H B. H- -H D. H H H H • H -H E. -H H H HICH T HHH F. H-arrow_forward
- Polylactic acid (shown below) is a biodegradable polymer used for food packaging. Identify the monomer(s) used in the production of this polymer using a condensation process.arrow_forwardDraw the product of the reaction shown below. Ignore small byproducts that would evaporate pleasearrow_forwardPoly(ethylene adipate) is a biodegradable polyester (shown below). Identify the type of polymerization process used in the production of this polymer.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
