
Fundamentals of Momentum, Heat, and Mass Transfer
6th Edition
ISBN: 9781118947463
Author: James Welty, Gregory L. Rorrer, David G. Foster
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 8, Problem 8.7P
Interpretation Introduction
Interpretation:
The ratio of the diameter of the cylinder for which the linear velocity profile assumption is accurate within 1 % of the actual velocity profile is to be determined.
Concept Introduction:
A control volume in a circular conduit is shown below as:
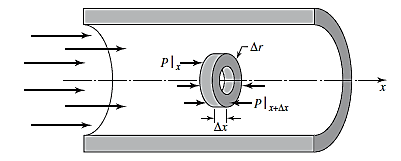
For this control volume, the expression for linear momentum in x direction is written as:
Here,
Newton’s law of viscosity is defined as:
Here,
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
5) Wet insulin crystals containing 32 g water per 100 g of dry insulin need to be dried in air to a
moisture level of 5 g water per 100 g of dry insulin. For your convenience, a graph of water
content of insulin vs. relative humidity and a psychrometric chart are provided below.
a) Determine the percentage of bound and unbound water in the wet crystals before drying.
b) Determine the relative humidity of the air to accomplish the drying to 5 g water/100 g dry
solids.
c) For drying with air at 20°C, what should be the moisture content of the air (g moisture/g dry
air)?
Water content (g/100 g dry solids)
40
30
20
10
Cefazolin sodium
tPA
20
40
Insulin
60
Relative humidity (%)
80
100
A power plant needs to evaporate 1500.0 lbm/h of water
at 40.0 °F at 1 atm. Utility superheated steam at 1200 °F
and 40 bar is available, but the steam cannot drop
below 20 bar and 700 °F.
Use the steam tables to determine the specific enthalpies of
the four streams. The reference for the enthalpies should
be water at the triple point. You may interpolate in Tables
B6. and B7, and can also use external, automated sources.
Determine the amount of superheated steam required to
accomplish the evaporation for a superheated steam outlet
of 700 °F and 20 bar. Assume no heat losses and use the
steam tables to determine enthalpies.
Recitation 11 Problem 1
400 kg/min of steam enters a steam turbine at 300 °C
and 80 bar through a 8.5-cm diameter line and exits at 100 °C
and 10.0 bar through a 5.5-cm line. The exiting stream may
be vapor, liquid, or "wet steam", a mist composed of saturated
water vapor and entrained liquid droplets.
Find how much power W (kW) is transferred from the turbine
to the steam? The answer can be positive or negative.
Neglect AE, but not AEK.
What percentage (%) of the total power is due to kinetic
energy changes?
Chapter 8 Solutions
Fundamentals of Momentum, Heat, and Mass Transfer
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A 30.0-g block of iron at 200.0°C is dropped into a liter of water in an insulated flask at 25.0°C and 1 atm. The specific enthalpy of iron is given by the expression Ĥ(J/g) = 17.3 T(°C).arrow_forwardRecitation 11 Problem 2 Eight fluid ounces (1 qt = fl 32 oz) of a beverage in a glass at 23.0 °C is to be cooled by adding ice and stirring. The properties of the beverage may be taken to be those of liquid water. The enthalpy of the ice relative to liquid water at the triple point is -348 kJ/kg. Estimate the mass of ice (g) that must melt to bring the liquid temperature to 4.0 °C, neglecting energy losses to the surroundings. (Note: For this isobaric batch process, the energy balance reduces to Q = AH)arrow_forwardA solid slab of 5.15 wt% agar gel at 278 K is 6.00 mm thick and contains a uniform concentration of urea of 0.2 kmol/m3. Diffusion is only in the direction through two parallel flat surfaces 6.00 mm apart. The slab is suddenly immersed in pure turbulent water so that the surface resistance can be assumed to be negligible; i.e, the convective coefficient hm is very large. The diffusivity of urea in the agar is 4.72e-10 m2/s. a) Calculate the concentration at the midpoint of the slab and (b) 1.50 mm from the surface after 19 h. c) if the thickness of the slab is halved, what would be the midpoint concentration in 19 h.arrow_forward
- The answer for the specific molar volume of nitrogen gas is 12.089x10^(-5) m^3/mol. How was this answer determined? You need to use the ideal gas law to determine the specific molar volume. Do not determine the third specific enthalpy. Also, how is the answer for (H3 specific enthalpy) of nitrogen gas (N2) equals to minus 1.26 KJ/mol???? The answer for the energy balance is minus 2320 KJ/s.arrow_forward220 20 g. 8 20 22 230 240 250 260 270 280 290 300 310 320 100 110 120 120 130 130 Enthalpy At Saturation (kJ/kg Dry Air) 140 150 160 170 180 190 200 210 Wet-Bulb se Seturation Temperature 80 8- ㄜ 30 40 50 60 70 Dry Bulb temperature (°C) g 80 90 100 110 120 10 10 330 60 340 350 360 370 380 390 400 170 990 00 50 09 Moisture Content (g/kg Dry Air) 30 40 70 60 100arrow_forward4) A bioproduct in an aqueous liquid is to be concentrated in a climbing film evaporator at a pressure of 200 mm Hg absolute. The maximum allowable entrained liquid is 0.001 kg liquid per kg of vapor. Equations to calculate the vapor pressure of water, liquid density, and vapor density are given in problem 10.3 of your textbook. Calculate the maximum allowable velocity of the vapor from the evaporator.arrow_forward
- 2) A protein is to be salted out of solution using ammonium sulfate. The protein solution is initially at 20 g/L. A plot of soluble protein vs. ammonium sulfate concentration is given below. a) What are the Cohn equation coefficients? b) Calculate the concentration of ammonium sulfate to precipitate out 99% of the protein. 4 2 y= -2.63x+7.54 R² = 1 In(S) 0 2 4 6 -2 -4 [Ammonium sulfate] (mol/L)arrow_forward3) A batch crystallization process was developed in a 2-L (working volume) reactor in the laboratory. The reactor impeller was 4.2 cm in diameter and operated at a speed of 715 rpm, which was the minimum speed required to fully suspend the crystals. You are asked to scale-up the crystallization process to an 850-L reactor. The fluid has the density and viscosity of water. a) Assuming geometric similarity between the small and large reactors, calculate the impeller diameter of the large reactor. b) Calculate the impeller speed in the large reactor if scaling up based on constant power per volume. c) Calculate the impeller speed in the large reactor if scaling up based on constant impeller tip speed. d) Calculate the impeller speed in the large reactor if scaling up based on minimum speed for full suspension of crystals.arrow_forward6) A wet cake of biological solids needs to be dried by blowing dry air across the top of the surface. Internal diffusion controls the mass transfer during drying. The moisture content of the cake is initially 60%, and the diffusion coefficient of water in the cake has been estimated to be 8.2 x 105 cm²/s. Estimate the cake depth that can be dried to a final moisture content of 5% in 24 h.arrow_forward
- Find v(t) fort > 0 in the circuit of Fig. below. Assume the switch has been open for a long time and is closed at t = 0. Calculate v (t) at t = 0.5. 10 V 202 ww +21 t=0 60 ww 13 F SVarrow_forwardYour client wants to separate a mixture of n-hexane, n-heptane, and methylcyclohexane (MCH) into nearly pure product streams, with n-heptane and methylcyclohexane being the light and heavy keys, respectively. However, n-heptane and methylcyclohexane have close boiling points, so normal fractionation could not be used. Assess how extractive distillation using aniline as a solvent can be used to separate the mixturearrow_forward2- An inlet water solution of 100 kg/h containing 0.010 wt fraction nicotine (A) in water is stripped with a kerosene stream of 200 kg/h containing 0.0005 wt fraction nicotine in a countercurrent-stage tower. The water and kerosene are essentially immiscible in each other. It is desired to reduce the concentration of the exit water to 0.0010 wt fraction nicotine. Determine the theoretical number of stages needed. The equilibrium data are as follows, with x the weight fraction of nicotine in the water solution and y in the kerosene: X y X y 0.001010 0.000806 0.00746 0.00682 0.00246 0.001959 0.00988 0.00904 0.00500 0.00454 0.0202 0.0185arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The