
ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259977596
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
thumb_up100%
Chapter 8, Problem 8.50P
Draw the products formed when each dihalide is treated with excess
a. 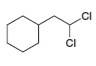 b.
b. 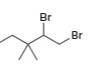 c.
c. 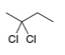 d.
d. 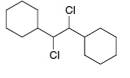
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Using line angle formulas, draw thestructures of and name four alkanes that have total of 7carbons, one of which is tertiary.Please explain this in detail and can you also explain how to approach a similar problem like this as well?
Using dashed line wedge projections drawthe indicated compounds and indicate whether thecompound you have drawn is R or S.(a) The two enantiomers of 2-chlorobutane. Can you please explain your steps and how you would approach a similar problem. Thank you!
5)
There are no lone pairs shown in the structure below. Please add in all lone pairs and then give the
hybridization scheme for the compound. (8)
10,11
7)
1.2.3
H
4
| 14
8)
COC
12
13
H
16
15
H7
9)
-
5.6
C
8
H
10)
H
1).
2)
3)_
11)
12)
13)
4)_
14)
5)
15)
16)
6)
Chapter 8 Solutions
ORGANIC CHEMISTRY
Ch. 8 - Problem 8.1 Label the and carbons in each alkyl...Ch. 8 - Problem 8.2 Classify each alkene in the following...Ch. 8 - Prob. 8.3PCh. 8 - Prob. 8.4PCh. 8 - Problem 8.5 Label each pair of alkenes as...Ch. 8 - Problem 8.6 Which alkene in each pair is more...Ch. 8 - Problem 8.7 Several factors can affect alkene...Ch. 8 - Prob. 8.8PCh. 8 - Prob. 8.9PCh. 8 - Prob. 8.10P
Ch. 8 - Prob. 8.11PCh. 8 - Problem 8.12 What alkenes are formed from each...Ch. 8 - Prob. 8.13PCh. 8 - Problem 8.14 What alkenes are formed from each...Ch. 8 - Problem 8.15 How does each of the following...Ch. 8 - Problem 8.16 Draw both the SN1 and E1 products of...Ch. 8 - Prob. 8.17PCh. 8 - Prob. 8.18PCh. 8 - Problem 8.19 Explain why...Ch. 8 - Prob. 8.20PCh. 8 - Problem 8.21 Draw the alkynes formed when each...Ch. 8 - Problem 8.22 Draw the products in each...Ch. 8 - Problem 8.23 Draw a stepwise mechanism for the...Ch. 8 - 8.24 Rank the alkenes shown in the ball-and-stick...Ch. 8 - Prob. 8.25PCh. 8 - 8.26 What is the major E2 elimination product...Ch. 8 - Prob. 8.27PCh. 8 - Prob. 8.28PCh. 8 - Prob. 8.29PCh. 8 - 8.30 Label each pair of alkenes as constitutional...Ch. 8 - Prob. 8.31PCh. 8 - Prob. 8.32PCh. 8 - Prob. 8.33PCh. 8 - For each of the following alkenes, draw the...Ch. 8 - Prob. 8.35PCh. 8 - Prob. 8.36PCh. 8 - Prob. 8.37PCh. 8 - What alkene is the major product formed from each...Ch. 8 - Prob. 8.39PCh. 8 - Prob. 8.40PCh. 8 - Pick the reactant or solvent in each part that...Ch. 8 - 8.42 In the dehydrohalogenation of...Ch. 8 - Prob. 8.43PCh. 8 - Prob. 8.44PCh. 8 - Prob. 8.45PCh. 8 - Prob. 8.46PCh. 8 - Prob. 8.47PCh. 8 - Prob. 8.48PCh. 8 - What alkyl chloride affords the following alkene...Ch. 8 - Draw the products formed when each dihalide is...Ch. 8 - Draw the structure of a dihalide that could be...Ch. 8 - Under certain reaction conditions, 2,...Ch. 8 - For which reaction mechanisms, SN1, SN2, E1 or...Ch. 8 - Draw the organic products formed in each...Ch. 8 - Prob. 8.55PCh. 8 - Draw all products, including stereoisomers, in...Ch. 8 - Draw all of the substitution and elimination...Ch. 8 - Prob. 8.58PCh. 8 - 8.59 Draw a stepwise, detailed mechanism for each...Ch. 8 - Draw the major product formed when...Ch. 8 - Draw a stepwise, detailed mechanism for the...Ch. 8 - Explain why the reaction of with gives ...Ch. 8 - Draw a stepwise detailed mechanism that...Ch. 8 - Prob. 8.64PCh. 8 - 8.65 Explain the selectivity observed in the...Ch. 8 - Prob. 8.66PCh. 8 - Prob. 8.67PCh. 8 - 8.68 (a) Draw all products formed by treatment of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
56. Global Positioning System. Learn more about the global positioning system and its uses. Write a short repo...
The Cosmic Perspective (8th Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
What are the cervical and lumbar enlargements?
Principles of Anatomy and Physiology
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The sum of the numbers in the name of isA. 11; B. 13; C. 10; D. 12; E. none of the other answers iscorrect. I believe the awnser should be E to this problem but the solution to this problem is D 12. I'm honestly unsure how that's the solution. If you can please explain the steps to this type of problem and how to approach a problem like this it would be greatly appreciated!arrow_forwardConsider the following data for phosphorus: g atomic mass 30.974 mol electronegativity 2.19 kJ electron affinity 72. mol kJ ionization energy 1011.8 mol kJ heat of fusion 0.64 mol You may find additional useful data in the ALEKS Data tab. Does the following reaction absorb or release energy? 2+ + (1) P (g) + e → P (g) Is it possible to calculate the amount of energy absorbed or released by reaction (1) using only the data above? If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (1): Does the following reaction absorb or release energy? 00 release absorb Can't be decided with the data given. yes no ☐ kJ/mol (²) P* (8) + + + e →>> P (g) Is it possible to calculate the amount of energy absorbed or released by reaction (2) using only the data above? If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (2): ☐ release absorb Can't be decided with the data given. yes no kJ/mol аarrow_forwardThe number of hydrogens in an alkyne that has a main chain of 14carbons to which are attached a cyclobutyl ring, a benzene ring, an–OH group, and a Br is A. 34; B. 35; C. 36; D. 24; E. 43arrow_forward
- Hello! I have a 500 Hz H-NMR for 1,5-bis-(4-methoxyphenyl)-penta-1,4-dien-3-one. I need to label the signals with the corresponding H's. Then, find out if the two alkenes are cis or trans by calculating the J values. I believe that I have the H-NMR labeled correctly, but not sure if I got the J values correct to determine if the two alkenes in the compound will make the compound cis or trans.arrow_forwardWhat is the only possible H-Sb-H bond angle in SbH3?arrow_forwardpls helparrow_forward
- Don't used hand raiting and don't used Ai solution and correct answerarrow_forwardDon't used hand raiting and don't used Ai solution and correct answerarrow_forwardPredict the product formed when the compound shown below undergoes a reaction with MCPBA in CH2Cl2. MCPBA is meta-chloroperoxybenzoic acid.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License