
(a)
Interpretation: All constitutional isomers formed in the given
Concept introduction: The removal of halide and neighboring
Answer to Problem 8.35P
All constitutional isomers formed in the given
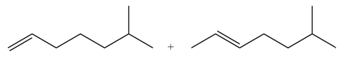
Explanation of Solution
The
In the given compound, two
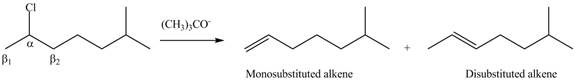
Figure 1
According to Zaitsev rule, more substituted alkene is obtained as a major product in
The product formed in the given reaction is shown in Figure 1.
(b)
Interpretation: All constitutional isomers formed in the given
Concept introduction: The removal of halide and neighboring
Answer to Problem 8.35P
All constitutional isomers formed in the given
Explanation of Solution
The
In the given compound, only one
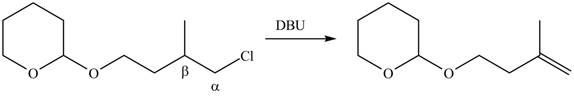
Figure 2
According to Zaitsev rule, more substituted alkene is obtained as a major product in
The product formed in the given reaction is shown in Figure 2.
(c)
Interpretation: All constitutional isomers formed in the given
Concept introduction: The removal of halide and neighboring
Answer to Problem 8.35P
All constitutional isomers formed in the given
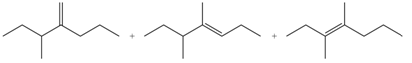
Explanation of Solution
The
In the given compound, three
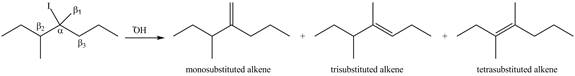
Figure 3
According to Zaitsev rule, more substituted alkene is obtained as a major product in
The product formed in the given reaction is shown in Figure 3.
(d)
Interpretation: All constitutional isomers formed in the given
Concept introduction: The removal of halide and neighboring
Answer to Problem 8.35P
All constitutional isomers formed in the given
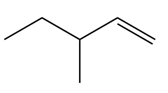
Explanation of Solution
In the given compound, only one
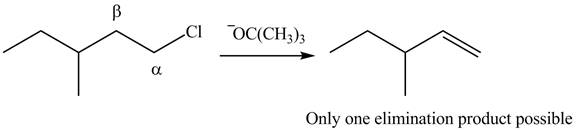
Figure 4
The product formed in the given reaction is shown in Figure 4.
(e)
Interpretation: All constitutional isomers formed in the given
Concept introduction: The removal of halide and neighboring
Answer to Problem 8.35P
All constitutional isomers formed in the given
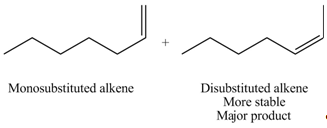
Explanation of Solution
The
In the given compound, two
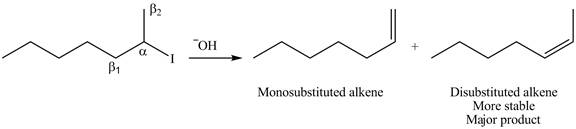
Figure 5
According to Zaitsev rule, more substituted alkene is obtained as a major product in
The product formed in the given reaction is shown in Figure 5.
(f)
Interpretation: All constitutional isomers formed in the given
Concept introduction: The removal of halide and neighboring
Answer to Problem 8.35P
All constitutional isomers formed in the given
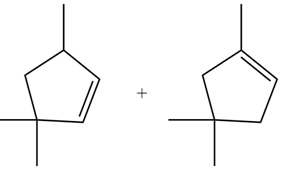 .
.
Explanation of Solution
The
In the given compound, two
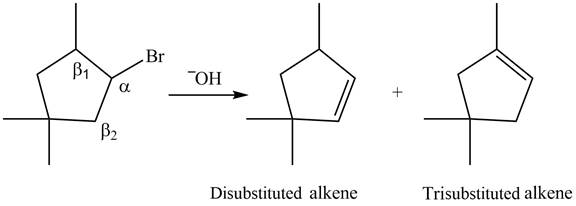
Figure 6
According to Zaitsev rule, more substituted alkene is obtained as a major product in
All constitutional isomers formed in the given
Want to see more full solutions like this?
Chapter 8 Solutions
Organic Chemistry-Package(Custom)
- Construct a molecular orbital energy-level diagram for BeH2. Sketch the MO pictures (schematic representation) for the HOMO and LUMO of BeH2 [Orbital Potential Energies, H (1s): -13.6 eV; Be (2s): -9.3 eV, Be (2p): -6.0 eV]arrow_forwardIndicate the isomers of the A(H2O)6Cl3 complex. State the type of isomerism they exhibit and explain it briefly.arrow_forwardState the formula of the compound potassium μ-dihydroxydicobaltate (III) tetraoxalate.arrow_forward
- Consider the reaction of the cyclopentanone derivative shown below. i) NaOCH2CH3 CH3CH2OH, 25°C ii) CH3!arrow_forwardWhat constitutes a 'reference material', and why does its utilization play a critical role in the chemical analysis of food products? Provide examples.arrow_forwardExplain what calibration is and why it is essential in relation to food analysis. Provide examples.arrow_forward
- The cobalt mu-hydroxide complex cobaltate(III) of potassium is a dinuclear complex. Correct?arrow_forwardThe cobalt mi-hydroxide complex cobaltate(III) of potassium is a dinuclear complex. Correct?arrow_forward3. Arrange the different acids in Exercise B # 2 from the strongest (1) to the weakest acid (10). 1. 2. (strongest) 3. 4. 5. 6. 7. 8. 9. 10 10. (weakest)arrow_forward
