
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 8, Problem 8.2P
Classify each
a.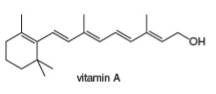 b.
b.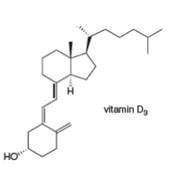
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.
Write the amididation reaction mechanism of p-aminophenol and acetic acid to produce acetaminophen please use arrows.
Name the following using IUPAC.
Chapter 8 Solutions
Organic Chemistry-Package(Custom)
Ch. 8 - Label the and carbons in each alkyl halide. Draw...Ch. 8 - Problem 8.2 Classify each alkene in the following...Ch. 8 - Prob. 8.3PCh. 8 - Prob. 8.4PCh. 8 - Problem 8.5 Label each pair of alkenes as...Ch. 8 - Prob. 8.6PCh. 8 - Problem 8.7 Several factors can affect alkene...Ch. 8 - Prob. 8.8PCh. 8 - Prob. 8.9PCh. 8 - Prob. 8.10P
Ch. 8 - Prob. 8.11PCh. 8 - What alkenes are formed from each alkyl halide by...Ch. 8 - Prob. 8.13PCh. 8 - Prob. 8.14PCh. 8 - Problem 8.15 How does each of the following...Ch. 8 - Prob. 8.16PCh. 8 - Prob. 8.17PCh. 8 - Prob. 8.18PCh. 8 - Prob. 8.19PCh. 8 - Problem 8.19 Explain why...Ch. 8 - Prob. 8.21PCh. 8 - Draw the alkynes formed when each dihalide is...Ch. 8 - Prob. 8.23PCh. 8 - Draw a stepwise mechanism for the following...Ch. 8 - 8.24 Rank the alkenes shown in the ball-and-stick...Ch. 8 - Prob. 8.26PCh. 8 - 8.26 What is the major E2 elimination product...Ch. 8 - Prob. 8.28PCh. 8 - Prob. 8.29PCh. 8 - Prob. 8.30PCh. 8 - Prob. 8.31PCh. 8 - PGF2 is a prostaglandin, a group of compounds that...Ch. 8 - Prob. 8.33PCh. 8 - Prob. 8.34PCh. 8 - Prob. 8.35PCh. 8 - Prob. 8.36PCh. 8 - Prob. 8.37PCh. 8 - Prob. 8.38PCh. 8 - Prob. 8.39PCh. 8 - Prob. 8.40PCh. 8 - What alkene is the major product formed from each...Ch. 8 - Prob. 8.42PCh. 8 - Prob. 8.43PCh. 8 - Prob. 8.44PCh. 8 - 8.42 In the dehydrohalogenation of...Ch. 8 - Prob. 8.46PCh. 8 - Prob. 8.47PCh. 8 - Prob. 8.48PCh. 8 - Prob. 8.49PCh. 8 - Prob. 8.50PCh. 8 - Draw the products of each reaction. a.c. b.d.Ch. 8 - Draw the structure of a dihalide that could be...Ch. 8 - Under certain reaction conditions, 2,...Ch. 8 - For which reaction mechanisms, SN1, SN2, E1 or...Ch. 8 - Draw the organic products formed in each reaction....Ch. 8 - Prob. 8.56PCh. 8 - Prob. 8.57PCh. 8 - Draw all products, including stereoisomers, in...Ch. 8 - Draw all of the substitution and elimination...Ch. 8 - Prob. 8.60PCh. 8 - 8.59 Draw a stepwise, detailed mechanism for each...Ch. 8 - Draw the major product formed when...Ch. 8 - Draw a stepwise, detailed mechanism for the...Ch. 8 - Explain why the reaction of with gives ...Ch. 8 - Draw a stepwise detailed mechanism that...Ch. 8 - Prob. 8.66PCh. 8 - 8.65 Explain the selectivity observed in the...Ch. 8 - Prob. 8.68PCh. 8 - Although dehydrohalogenation occurs with anti...Ch. 8 - 8.68 (a) Draw all products formed by treatment of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
2. Why is it that the range of resting blood pressures of humans is best represented by a bell-shaped curve co...
Human Biology: Concepts and Current Issues (8th Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forwardName the following molecules with IUpacarrow_forward
- What is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forward
- Please help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forwardProvide solutionsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY