
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 8.16P
Draw both the SN1 and E1 products of each reaction.
a.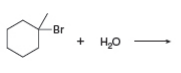 b.
b.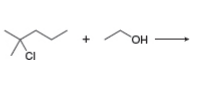
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please correct answer and don't use hand rating
2. Draw mechanisms for the following reactions.
mg
Et
CO₂Hot
H30t
Et
0
Please correct answer and don't use hand rating
Chapter 8 Solutions
Organic Chemistry
Ch. 8 - Problem 8.1 Label the and carbons in each alkyl...Ch. 8 - Problem 8.2 Classify each alkene in the following...Ch. 8 - Prob. 8.3PCh. 8 - Prob. 8.4PCh. 8 - Problem 8.5 Label each pair of alkenes as...Ch. 8 - Problem 8.6 Which alkene in each pair is more...Ch. 8 - Problem 8.7 Several factors can affect alkene...Ch. 8 - Prob. 8.8PCh. 8 - Prob. 8.9PCh. 8 - Prob. 8.10P
Ch. 8 - Prob. 8.11PCh. 8 - Problem 8.12 What alkenes are formed from each...Ch. 8 - Prob. 8.13PCh. 8 - Problem 8.14 What alkenes are formed from each...Ch. 8 - Problem 8.15 How does each of the following...Ch. 8 - Problem 8.16 Draw both the SN1 and E1 products of...Ch. 8 - Prob. 8.17PCh. 8 - Prob. 8.18PCh. 8 - Problem 8.19 Explain why...Ch. 8 - Prob. 8.20PCh. 8 - Problem 8.21 Draw the alkynes formed when each...Ch. 8 - Problem 8.22 Draw the products in each...Ch. 8 - Problem 8.23 Draw a stepwise mechanism for the...Ch. 8 - 8.24 Rank the alkenes shown in the ball-and-stick...Ch. 8 - Prob. 8.25PCh. 8 - 8.26 What is the major E2 elimination product...Ch. 8 - Prob. 8.27PCh. 8 - Prob. 8.28PCh. 8 - Prob. 8.29PCh. 8 - 8.30 Label each pair of alkenes as constitutional...Ch. 8 - Prob. 8.31PCh. 8 - Prob. 8.32PCh. 8 - Prob. 8.33PCh. 8 - For each of the following alkenes, draw the...Ch. 8 - Prob. 8.35PCh. 8 - Prob. 8.36PCh. 8 - Prob. 8.37PCh. 8 - What alkene is the major product formed from each...Ch. 8 - Prob. 8.39PCh. 8 - Prob. 8.40PCh. 8 - Pick the reactant or solvent in each part that...Ch. 8 - 8.42 In the dehydrohalogenation of...Ch. 8 - Prob. 8.43PCh. 8 - Prob. 8.44PCh. 8 - Prob. 8.45PCh. 8 - Prob. 8.46PCh. 8 - Prob. 8.47PCh. 8 - Prob. 8.48PCh. 8 - What alkyl chloride affords the following alkene...Ch. 8 - Draw the products formed when each dihalide is...Ch. 8 - Draw the structure of a dihalide that could be...Ch. 8 - Under certain reaction conditions, 2,...Ch. 8 - For which reaction mechanisms, SN1, SN2, E1 or...Ch. 8 - Draw the organic products formed in each...Ch. 8 - Prob. 8.55PCh. 8 - Draw all products, including stereoisomers, in...Ch. 8 - Draw all of the substitution and elimination...Ch. 8 - Prob. 8.58PCh. 8 - 8.59 Draw a stepwise, detailed mechanism for each...Ch. 8 - Draw the major product formed when...Ch. 8 - Draw a stepwise, detailed mechanism for the...Ch. 8 - Explain why the reaction of with gives ...Ch. 8 - Draw a stepwise detailed mechanism that...Ch. 8 - Prob. 8.64PCh. 8 - 8.65 Explain the selectivity observed in the...Ch. 8 - Prob. 8.66PCh. 8 - Prob. 8.67PCh. 8 - 8.68 (a) Draw all products formed by treatment of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Describe the role and impact of microbes on the earth.
Microbiology Fundamentals: A Clinical Approach
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
The validity of a scientific law.
Physical Universe
2. Why is it that the range of resting blood pressures of humans is best represented by a bell-shaped curve co...
Human Biology: Concepts and Current Issues (8th Edition)
What were the major microbiological interests of Martinus Beijerinck and Sergei Winogradsky? It can be said tha...
Brock Biology of Microorganisms (15th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Convert the following structures into a chair representation. Then conduct a chair flip. Cl a. b. C\.... оarrow_forwardAktiv Learning App Cengage Digital Learning Part of Speech Table for Assign x o Mail-Karen Ento-Outlook * + app.aktiv.com Your Aktiv Learning trial expires on 02/06/25 at 01:15 PM Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 17 of 30 Drawing Arrows heat 4 O M B D 5x H H Und Settings H Done :0: H Jararrow_forwardConvert the following chairs into ring representations: a. Brz b.arrow_forward
- Drawing Arrows 1 I I 1 heat 1 51 MO + Drag To Und Settings Done 0 0 Jan 31 3:5arrow_forwardDon't used hand raitingarrow_forwardGramicidin A can adopt more than one structure; NMR spectroscopy has revealed an “end-to-end” dimer form, and x-ray crystallography has revealed an “anti-parallel double- helical” form. Briefly outline and describe an experimentalapproach/strategy to investigate WHICH configuration (“end-to-end dimer” vs “anti-paralleldouble helical”) gramicidin adopts in an actual lipid bilayer.arrow_forward
- Don't used hand raitingarrow_forwardCHEM2323 Problem 2-24 Tt O e: ל Predict the product(s) of the following acid/base reactions. Draw curved arrows to show the formation and breaking of bonds. If the bonds needed are not drawn out, you should redraw them. + BF3 (a) (b) HI + (c) OH -BF Problem 2-25 Use curved arrows and a proton (H+) to draw the protonated form of the following Lewis bases. Before starting, add all missing lone pairs. (a) (b) :0: (c) N 1 CHEM2323 PS CH02 Name:arrow_forwardCHEM2323 Problem 2-26 Tt O PS CH02 Name: Use the curved-arrow formalism to show how the electrons flow in the resonance form on the left to give the one on the right. (Draw all lone pairs first) (a) NH2 NH2 + (b) Problem 2-27 Double bonds can also act like Lewis bases, sharing their electrons with Lewis acids. Use curved arrows to show how each of the following double bonds will react with H-Cl and draw the resulting carbocation. (a) H2C=CH2 (b) (c) Problem 2-28 Identify the most electronegative element in each of the following molecules: (a) CH2FCI F Problem 2-29 (b) FCH2CH2CH2Br (c) HOCH2CH2NH2 (d) CH3OCH2Li F 0 0 Use the electronegativity table in Figure 2.3 to predict which bond in the following pairs is more polar and indicate the direction of bond polarity for each compound. (a) H3C-Cl or Cl-CI (b) H3C-H or H-CI (c) HO-CH3 or (CH3)3Si-CH3 (d) H3C-Li or Li-OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License