
(a)
Interpretation: The plot for the given seven ionization energies (IE) for the given atom, the shell that has electrons corresponds to the respective IE and the reason for presence of the break in graph should be determined.
Concept Introduction:
Cation: Removal of electron from the atom results to form positively charged ion called cation.
Anion: Addition of electron to atom results to form negatively charged ion called anion.
The net charge present in the element denotes the presence or absence of electrons in the element.
First ionization energy:
The ionization energy is the minimum energy required to remove the electron from an isolated atom which is in the gaseous state results to give gaseous ion with one positive charge.
Second ionization:
Repeating the same process that is removal of another electron that is second electron from the resulting ion of first ionization is called second ionization.
Third ionization energy:
Removal of electron from ion that results from the second ionization is called third ionization which results to give ion with three positive charges which shows, three electrons gets removed from the atom and the energy associated with it is called third ionization energy.
To plot: The graph considering the given ionization energies for the given element.
(a)
Answer to Problem 8.109QP
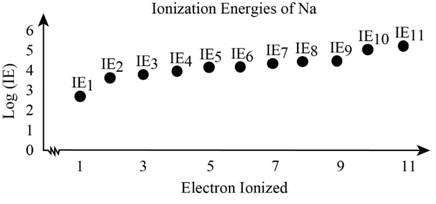
Analyze the given data and plot the data for the given element.
Explanation of Solution
The graph that shows the ionization energies for the given elements is drawn considering the given data.
The ionization energy increases since it involves removal of electrons such the successive removal of electrons requires more energy compared to the previous as core electrons tends to tightly bound with the nucleus.
(b)
Interpretation: The plot for the given seven ionization energies (IE) for the given atom, the shell that has electrons corresponds to the respective IE and the reason for presence of the break in graph should be determined.
Concept Introduction:
Cation: Removal of electron from the atom results to form positively charged ion called cation.
Anion: Addition of electron to atom results to form negatively charged ion called anion.
The net charge present in the element denotes the presence or absence of electrons in the element.
First ionization energy:
The ionization energy is the minimum energy required to remove the electron from an isolated atom which is in the gaseous state results to give gaseous ion with one positive charge.
Second ionization:
Repeating the same process that is removal of another electron that is second electron from the resulting ion of first ionization is called second ionization.
Third ionization energy:
Removal of electron from ion that results from the second ionization is called third ionization which results to give ion with three positive charges which shows, three electrons gets removed from the atom and the energy associated with it is called third ionization energy.
To determine: The orbital that contains the electron with respect to the energy.
(b)
Explanation of Solution
Examining the graph shows that first the valence electron in the given element gets removed which present in the
The orbital that contains electrons with respect to the given energy is determined as above.
(c)
Interpretation: The plot for the given seven ionization energies (IE) for the given atom, the shell that has electrons corresponds to the respective IE and the reason for presence of the break in graph should be determined.
Concept Introduction:
Cation: Removal of electron from the atom results to form positively charged ion called cation.
Anion: Addition of electron to atom results to form negatively charged ion called anion.
The net charge present in the element denotes the presence or absence of electrons in the element.
First ionization energy:
The ionization energy is the minimum energy required to remove the electron from an isolated atom which is in the gaseous state results to give gaseous ion with one positive charge.
Second ionization:
Repeating the same process that is removal of another electron that is second electron from the resulting ion of first ionization is called second ionization.
Third ionization energy:
Removal of electron from ion that results from the second ionization is called third ionization which results to give ion with three positive charges which shows, three electrons gets removed from the atom and the energy associated with it is called third ionization energy.
To determine: The reason for the breaks in the curve of the plot for given element.
(c)
Explanation of Solution
The break in the curve of the plot explains that removal of electrons from one energy level to other level that is break is appeared two times one with going from third to 2nd level and the other from 2nd to the first energy level.
Want to see more full solutions like this?
Chapter 8 Solutions
Connect for Chemistry
- AE>AE₁ (Y/N) AE=AE₁ (Y/N) AEarrow_forwardTreatment of 2-phenylpropan-2-amine with methyl 2,4-dibromobutanoate in the presence of a nonnucleophilic base, R3N, involves two successive SN2 reactions and gives compound A. ? NH2 Br Br Propose a structural formula for compound A. You do not have to explicitly draw H atoms. You do not have to consider stereochemistry. In cases where there is more than one answer, just draw one. R3N C14H19NO2 + 2 R3NH*Br Aarrow_forwardCorrectly name this compound using the IUPAC naming system by sorting the components into the correct order. Br IN Ν Harrow_forwardHow is the radical intermediate for this structure formed? Can you please draw arrows from the first radical to the resonance form that would result in this product? I'm lost.arrow_forwardPart VI. (a) calculate the λ max of the compound using woodward - Fieser rules. (b) what types of electronic transitions are present in the compound? (c) what are the prominent peaks in the IR spectrum of the compound?arrow_forwardDon't used Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forward↑ 0 Quiz List - RCC430M_RU05 X Aktiv Learning App × Qdraw resonance structure ×Q draw resonance structure xb My Questions | bartleby ×+ https://app.aktiv.com Draw a resonance structure of pyrrole that has the same number of pi bonds as the original structure. Include all lone pairs in your structure. + N H a 5 19°F Cloudy Q Search Problem 12 of 15 Atoms, Bonds and Rings Charges and Lone Pairs myhp हजु Undo Reset Remove Done Submit Drag To Pan 2:15 PM 1/25/2025arrow_forwardDon't used hand raitingarrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





