
Concept explainers
Interpretation:
The pH values after the addition of each proportion of the base to the acid is to be determined. Also, the titration curve needs to be drawn.
Concept introduction:
Titration curve is drawn to determine the change in pH of an acid or base with respect to the added volume of base or acid to it.
The titration curve can be drawn between a strong/weak acid and strong/weak base. The change in pH shows different patterns for different combinations of acids and bases.
Answer to Problem 69E
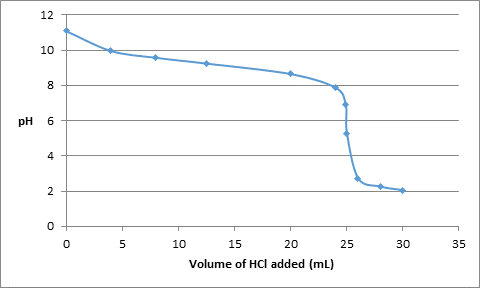
The change in pH due to addition of HCl is represented as follows:
| Volume of HCl added (mL) | pH |
| 0 | 11.1 |
| 4.0 | 9.97 |
| 8.0 | 9.58 |
| 12.5 | 9.25 |
| 20.0 | 8.65 |
| 24.0 | 7.87 |
| 24.9 | 6.9 |
| 25.0 | 5.28 |
| 26.0 | 2.71 |
| 28.0 | 2.24 |
| 30.0 | 2.04 |
The titration curve can be drawn as follows:
Explanation of Solution
Initial pH of the analyte solution; Ammonia is a weak base that forms an equilibrium when dissolved in water. The equilibrium is as follows.
The molarity of ammonia at 0 mL HCl is 0.100 M. The ICE table for its dissociation can be represented as follows:
| Reaction | Ammonia | Ammonium | OH- |
| Initial | 0.1 | 0 | 0 |
| Change | -x | +x | +x |
| Equilibrium | (0.1-x) | x | x |
The value of x can be calculated by solving the equation as follows:
Then the pH of the initial solution can be determined.
Thus, pH of solution when 0 mL of acid is added is 11.1.
Addition of
When 4.0 mL of acid is added the number of moles of ammonia and HCl can be calculated from their molarity and volume as follows:
The ICE table can be represented as follows:
| Reaction | Ammonia | HCl | Ammonium chloride |
| Initial | 0.0025 | 0.0004 | 0 |
| Change | -0.0004 | -0.0004 | +0.0004 |
| Equilibrium | 0.0021 | 0 | 0.0004 |
The pH can be calculated using the Henderson-Hesselbalch equation as follows:
Now,
In the Henderson-Hasselbalch equation, the pKa is used. therefore, the pKa for ammonia need to be calculated using its pKb.
Applying the Henderson-Hasselbalch equation,
Thus, the pH of the solution is 9.97.
Addition of
Total amount of ammonia to be neutralized
Amount of acid added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Ammonia | HCl | Ammonium chloride |
| Initial | 0.0025 | 0.0008 | 0 |
| Change | -0.0008 | -0.0008 | +0.0008 |
| Equilibrium | 0.0017 | 0 | 0.0008 |
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of ammonia to be neutralized
Amount of acid added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Ammonia | H+ | Ammonium chloride |
| Initial | 0.0025 | 0 | 0 |
| Change | -0.00125 | -0.00125 | +0.00125 |
| Equilibrium | 0.00125 | 0 | 0.00125 |
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of ammonia to be neutralized
Amount of acid added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Ammonia | H+ | Ammonium |
| Initial | 0.0025 | 0 | 0 |
| Change | -0.002 | -0.002 | +0.002 |
| Equilibrium | 0.0005 | 0 | 0.002 |
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of ammonia to be neutralized
Amount of acid added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Ammonia | HCl | Ammonium chloride |
| Initial | 0.0025 | 0 | 0 |
| Change | -0.0024 | -0.0024 | 0.0024 |
| Equilibrium | 0.0001 | 0 | 0.0024 |
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of ammonia to be neutralized
Amount of acid added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Ammonia | HCl | Ammonium chloride |
| Initial | 0.0025 | 0 | 0 |
| Change | -0.00249 | -0.00249 | -0.00249 |
| Equilibrium | 0.00001 | 0 | 0.00249 |
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of ammonia to be neutralized
Amount of acid added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Ammonia | H+ | Ammonium |
| Initial | 0.0025 | 0 | 0 |
| Change | -0.0025 | -0.0025 | -0.0025 |
| Equilibrium | 0.0000 | 0 | 0.0025 |
From the number of moles of ammonium ion and total volume that is 0.05 L, the molarity of ammonium chloride solution can be calculated as follows:
The ammonium ion from salt can react with water to give back ammonia as follows:
The ICE table can be prepared as follows:
The acid dissociation constant can be represented as follow:
From the ICE table,
Since, the value of acid dissociation constant is very low thus; value of x can be neglected from denominator.
This is the concentration of hydrogen ion in the solution.
The pH can be calculated as follows:
Addition of 26 mL of the acid:
The number of moles of ammonia and acid will be:
The reaction is represented as follows:
| Reaction | Ammonia | HCl | Ammonium choride |
| Initial | 0.0025 | 0.0026 | 0 |
| Change | -0.0025 | -0.0025 | +0.0025 |
| Equilibrium | 0 | 0.0001 | 0.0025 |
The pH depends only on the concentration of HCl as it is a strong acid.
The molarity of HCl using total volume of the solution will be:
The pH can be calculated as follows:
Addition of 28 mL of acid:
The number of moles of each species will be:
The reaction is represented as follows:
| Reaction | Ammonia | HCl | Ammonium choride |
| Initial | 0.0025 | 0.0028 | 0 |
| Change | -0.0025 | -0.0025 | +0.0025 |
| Equilibrium | 0 | 0.0003 | 0.0025 |
The pH depends only on the concentration of HCl as it is a strong acid.
The molarity of HCl using total volume of the solution will be:
The pH can be calculated as follows:
Addition of
The number of moles of ammonia and HCl can be calculated as follows:
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Ammonia | H+ | Ammonium | OH- |
| Initial | 0.0025 | 0.003 | 0 | 0 |
| Change | -0.0025 | -0.0025 | 0 | 0 |
| Equilibrium | 0 | 0.0005 | 0 | 0 |
Concentration of hydrogen ion
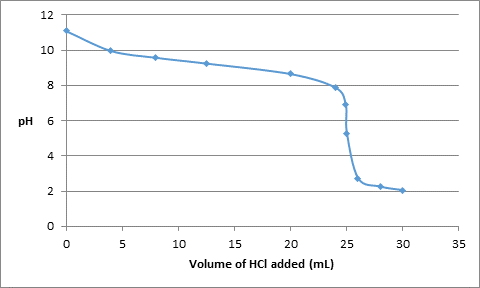
The data obtained from the above calculations will be:
| Volume of HCl added (mL) | pH |
| 0 | 11.1 |
| 4.0 | 9.97 |
| 8.0 | 9.58 |
| 12.5 | 9.25 |
| 20.0 | 8.65 |
| 24.0 | 7.87 |
| 24.9 | 6.9 |
| 25.0 | 5.28 |
| 26.0 | 2.71 |
| 28.0 | 2.24 |
| 30.0 | 2.04 |
The titration curve can be drawn as follows:
Want to see more full solutions like this?
Chapter 8 Solutions
EBK CHEMICAL PRINCIPLES
- Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.200 M HClarrow_forwardCalculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.000259 M HClO4arrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forward
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forwardCan I please get help with this?arrow_forwardCan I please get help with this?arrow_forward
- Use the Henderson-Hasselbalch equation to calculate pH of a buffer containing 0.050M benzoic acidand 0.150M sodium benzoate. The Ka of benzoic acid is 6.5 x 10-5arrow_forwardA. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forward
- Can I please get help with this.arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





