
Concept explainers
How many
How many yield 2,3-dimethylbutane?
How many yield methylcyclobutane?
Interpretation:
The number of alkenes which are required to produce the given alkanes on catalytic hydrogenation is to be determined.
Concept introduction:
On catalytic hydrogenation, alkenes get converted to the corresponding alkanes with the same number of carbon atoms.
In hydrogenation reaction, one hydrogen atom gets attached to each of the double bonded carbon atoms.
To accelerate the rate of hydrogenation, the metal catalyst provides an alternative pathway involving low activation energy steps.
The alkane that is formed contains two hydrogen atoms more than the corresponding alkene and these two hydrogen atoms are added on the adjacent carbon atoms in the alkene.
Answer to Problem 25P
Solution:
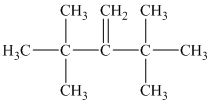
a) Only one alkene can produce
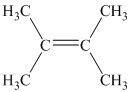
b) Two alkenes can produce
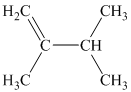
c) Three alkenes can produce methylcyclobutane upon catalytic hydrogenation.
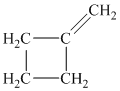
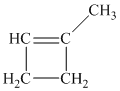
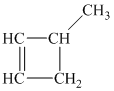
Explanation of Solution
a) The structure of
Hydrogenation of alkenes adds one hydrogen atom on each carbon atom of the double bonded carbon atoms. So in the alkane, these two carbon atoms (previously double bonded) should be attached to at least one hydrogen atom each.
In
The alkane structure is symmetric, and carbon atoms
b) The structure of
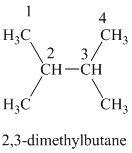
The hydrogen of alkenes adds one hydrogen atom on each carbon atom of the double bonded carbon atoms. So in the alkane, these two carbon atoms (previously double bonded) should be attached to at least one hydrogen atom each.
In
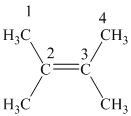
The
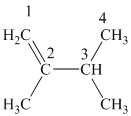
The alkane structure is symmetric, and the carbon atoms
Hence, two alkenes can produce
c) The structure of methylcyclobutane is shown below:
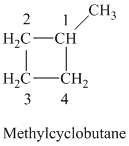
The hydrogen of alkenes adds one hydrogen atom on each carbon atom of the double bonded carbon atoms. So in the alkane, these two carbon atoms (previously double bonded) should be attached to one at least one hydrogen atom each.
In methylcyclobutane, the
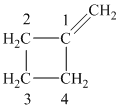
The
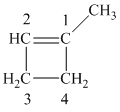
The
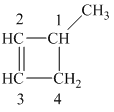
The alkane structure is symmetric. This means, there cannot be any other distinct alkene structure which would produce methylcyclobutane upon catalytic hydrogenation.
Hence, three alkenes can produce methylcyclobutane upon catalytic hydrogenation.
Want to see more full solutions like this?
Chapter 8 Solutions
Organic Chemistry - Standalone book
- a. Explain Why electron withdrawing groupe tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures 6. Explain why -ll is an ortho -pura drccton evon though chlorine has a very High Electronegativityarrow_forwardC. Ν Harrow_forwarda. H3C. N H3C CH3 HCNarrow_forward
- ол 2. восцапан (46:00) Curtius rearrangment 1. NaN3, heat -OHarrow_forwardQuestion 1. Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers.arrow_forwardElectrochemistry. Briefly describe the Donnan potential.arrow_forward
- Indicate what the Luther equation is used for?arrow_forwardIndicate one aspect that benefits and another that makes it difficult to use the hydroquinone electrode to measure pH.arrow_forwardAt an electrified interface according to the Gouy-Chapman model, what types of interactions do NOT occur between the ions and the solvent according to this theory?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


