
EBK ORGANIC CHEMISTRY AS A SECOND LANGU
4th Edition
ISBN: 8220102737037
Author: Klein
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7.6, Problem 7.37P
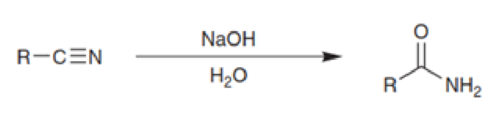
Based on everything we have just seen, propose a mechanism for the hydration of a nitrile under basic conditions:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please answer with explanations and make it easy to read
Propose a synthesis of the following molecule, starting with cyclohexanone and using any other
reagents necessary:
Reaction of p-nitroaniline with sodium nitrite and hydrochloric acid at 0°C,
followed by treatment with N,N-diethylaniline.
Chapter 7 Solutions
EBK ORGANIC CHEMISTRY AS A SECOND LANGU
Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Propose a plausible mechanism for each of the...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...Ch. 7.3 - Predict the major product in each of the following...
Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.3 - Identify the reagents you would use to achieve...Ch. 7.4 - Predict the major products for each of the...Ch. 7.4 - Predict the major products for each of the...Ch. 7.4 - Predict the major products for each of the...Ch. 7.5 - Prob. 7.23PCh. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - Identify the reagents you would use to make each...Ch. 7.5 - In the space provided, draw a mechanism for each...Ch. 7.5 - In the space provided, draw a mechanism for each...Ch. 7.5 - Prob. 7.33PCh. 7.5 - Prob. 7.34PCh. 7.5 - Prob. 7.35PCh. 7.5 - Prob. 7.36PCh. 7.6 - Based on everything we have just seen, propose a...Ch. 7.6 - Prob. 7.39PCh. 7.6 - Prob. 7.40PCh. 7.6 - Prob. 7.41PCh. 7.6 - Propose a mechanism for the following reaction:Ch. 7.6 - Prob. 7.44PCh. 7.6 - Prob. 7.45PCh. 7.6 - Prob. 7.46PCh. 7.7 - Prob. 7.48PCh. 7.7 - Prob. 7.49PCh. 7.7 - Prob. 7.50PCh. 7.7 - Prob. 7.51PCh. 7.7 - Prob. 7.52PCh. 7.7 - Prob. 7.53PCh. 7.7 - Prob. 7.55PCh. 7.7 - Prob. 7.56PCh. 7.7 - Prob. 7.57PCh. 7.7 - Prob. 7.58PCh. 7.7 - Prob. 7.59PCh. 7.7 - Prob. 7.60PCh. 7.7 - Prob. 7.61PCh. 7.7 - Prob. 7.62PCh. 7.7 - Prob. 7.63PCh. 7.7 - Prob. 7.64PCh. 7.7 - Prob. 7.65P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Complete and balance each equation. If no reaction occurs, write NO REACTION. a. KI(aq)+BaS(aq) b. K2SO4(aq)+Ba...
Introductory Chemistry (6th Edition)
a. Draw the resonance forms for SO2 (bonded OSO). b. Draw the resonance forms for ozone (bonded OOO). c. Sulfur...
Organic Chemistry (9th Edition)
For Practice 1.1
Is each change physical or chemical? Which kind of property (chemical or physical) is demonst...
Principles of Chemistry: A Molecular Approach (3rd Edition)
4.1 Write the symbols for the following elements.
a. copper
b. platinum
c. calcium
d. manganese
e. Iron
...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
PRACTICE 1.3 The melting point of table salt is 1474oF. What temperature is this on the Celsius and Kelvin scal...
CHEMISTRY-TEXT
In qualitative analysis, Ca2+ and Ba2+ are separated from Na+, K+, and Mg2+ by adding aqueous (NH4)2CO3 to a so...
General Chemistry: Atoms First
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Reaction of p-nitroaniline with sodium nitrite and hydrochloric acid at 0 °C, followed by treatment with N,N-diethylaniline.arrow_forwardIn an aqueous solution containing sodium bicarbonate, aniline reacts quickly withbromine to give 2,4,6-tribromoaniline. Nitration of aniline requires very strong conditions,however, and the yields (mostly m-nitroaniline) are poor.(a) What conditions are used for nitration, and what form of aniline is present under theseconditions?arrow_forward19.24b Show how you would synthesize the below compound from 1,2,5-pentanetriol OH OH HO (b)arrow_forward
- One step in the urea cycle for ridding the body of ammonia is the conversion of argininosuccinate to the amino acid arginine plus fumarate. Propose a mechanism for the reaction, and show the structure of arginine.arrow_forwardFollowing is a synthesis for toremifene, a nonsteroidal estrogen antagonist whose structure is closely related to that of tamoxifen. (a) This synthesis makes use of two blocking groups, the benzyl (Bn) group and the tetrahydropyranyl (THP) group. Draw a structural formula of each group and describe the experimental conditions under which it is attached and removed. (b) Discuss the chemical logic behind the use of each blocking group in this synthesis. (c) Propose a mechanism for the conversion of D to E. (d) Propose a mechanism for the conversion of F to toremifene. (e) Is toremifene chiral? If so, which of the possible stereoisomers are formed in this synthesis?arrow_forwardThe mechanism for acidic hydrolysis of a nitrile resembles the basic hydrolysis, exceptthat the nitrile is first protonated, activating it toward attack by a weak nucleophile (water).Under acidic conditions, the proton transfer (tautomerism) involves protonation on nitrogen followed by deprotonation on oxygen. Propose a mechanism for the acid-catalyzedhydrolysis of benzonitrile to benzamide.arrow_forward
- The two most general amine syntheses are the reductive amination of carbonyl compounds and the reduction of amides.Show how these techniques can be used to accomplish the following syntheses.(a) benzoic acid S benzylamine (b) benzaldehyde S benzylamine(c) pyrrolidine S N@ethylpyrrolidine (d) cyclohexanone S N@cyclohexylpyrrolidine(e) HOOC¬(CH2)3 ¬COOH S pentane@1,5@diamine (cadaverine)arrow_forward6arrow_forwardHow are following obtained: (i) Toluene from phenol (ii) Phenol from Aniline.arrow_forward
- 1. Cetrizine is a nonsedating antihistamine. The first step in a synthesis of cetirizine involves the following Grignard reaction. Give the major product and propose a reasonable mechanism for the following reaction: H CI 1) 2) HCI, H₂O MgBr diethyl etherarrow_forwardShow how to synthesize the following amines from the indicated starting materials byacylation–reduction.(a) N-butylpiperidine from piperidinearrow_forwardiv)Propose a mechanism (curved arrow formalism) for the reaction of the synthesis of a sulfonamide derivative from sulfathiazolearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License