
Air enters the evaporator section of a window air conditioner at 100 kPa and 27°C with a volume flow rate of 6 m3/min. The refrigerant-134a at 120 kPa with a quality of 0.3 enters the evaporator at a rate of 2 kg/min and leaves as saturated vapor at the same pressure. Determine the exit temperature of the air and the rate of entropy generation for this process, assuming (a) the outer surfaces of the air conditioner are insulated and (b) heat is transferred to the evaporator of the air conditioner from the surrounding medium at 32°C at a rate of 30 kJ/min.
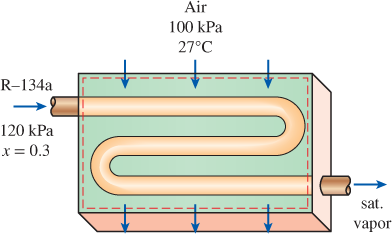
FIGURE P7–180
a)
The exit temperature of air and the entropy generated during the process.
Answer to Problem 180RP
The exit temperature of air is
The entropy generated during the process is
Explanation of Solution
Write the expression to calculate the initial enthalpy of the refrigerant.
Here, initial enthalpy is
Write the expression to calculate the initial entropy of the refrigerant.
Here, initial entropy is
Write the expression to calculate the mass flow rate of air.
Here, mass flow rate of air is
Write the expression for the mass balance of the system.
Here, mass flow rate into the control system is
Write the expression for the energy balance equation for closed system.
Here, rate of energy transfer into the control volume is
Write the expression for the rate of entropy balance for the system.
Here, rate of entropy in the system is
Conclusion:
From Table A-12, “Saturated refrigerant-134a-Pressure table”, Obtain the following properties at saturated pressure of 120 kPa
Saturated liquid enthalpy,
Evaporated enthalpy,
Saturated vapor enthalpy,
Saturated vapor entropy,
Saturated liquid entropy,
Evaporated entropy,
Substitute
Substitute
Refrigerant –134a enters and leaves at the same pressure. Hence,
From Table A-1E, “the molar mass, gas constant and critical–point properties table”, select the gas constant of air at room temperature as
Substitute
Substitute
Here, mass flow rate of refrigerant is
Substitute
Here, mass flow rate of air is
Substitute
From the Table A-2, “Ideal-gas specific heats of various common gases”, select the value of the specific heat at constant pressure value of air as
Substitute 27 C for
Hence, the exit temperature of air is
For the steady flow system, change of entropy in the system is zero.
Substitute
Substitute
Hence, the entropy generated during the process is
b)
The exit temperature of air and the entropy generated during the process.
Answer to Problem 180RP
The exit temperature of air is
The entropy generated during the process is
Explanation of Solution
Write the expression for the energy balance equation for closed system.
Here, rate of energy transfer into the control volume is
Write the expression for the rate of entropy balance for the system.
Here, rate of entropy in the system is
Conclusion:
Substitute
Here, rate of heat gain from the surrounding is
Substitute 27 C for
Hence, the exit temperature of air is
For the steady flow system, change of entropy in the system is zero.
Substitute
Substitute
Hence, the entropy generated during the process is
Want to see more full solutions like this?
Chapter 7 Solutions
Thermodynamics: An Engineering Approach
- Refrigerant-134a enters a diffuser steadily as saturated vapor at 700 kPa with a velocity of 150 m/s, and it leaves at 800 kPa and 50°C. The refrigerant is gaining heat at a rate of 5kJ/s as it passes through the diffuser. If the exit area is 70 percent greater than the inlet area, determine (a) the exit velocity and (b) the mass flow rate of the refrigerant.arrow_forwardWater vapor enters a turbine with a mass flow rate of 3 kg/s, and at a temperature and pressure of 500°C and 1 MPa, respectively. The heat loss inside the turbine is 250 kW and the steam leaves the turbine at a temperature and pressure of 150°C and 100 kPa. Neglect any changes in the velocity or the elevation. The work output of the turbine is used to operate a heat pump whose COP value is 2. Determine the rate of heat removal from the sink (1) and the rate of heat rejection to the source (2) of this heat pump. a. 3715 kW (removal), 1857.5 kW (rejection) b. 1857.5 kW (removal), 3500 kW (rejection) c. 1857.5 kW (removal), 3715 kW (rejection) d. 1500 kW (removal), 3715 kW (rejection)arrow_forwardAn adiabatic capillary tube is used in some refrigeration systems to drop the pressure of the refrigerant from the condenser level to the evaporator level. R-134a enters the capillary tube as a saturated liquid at 65.9 oC, and leaves at -12 oC. Determine the rate of entropy generation in the capillary tube for a mass flow rate of 0.83 kg/s. s1 (kJ/kgK) Format : 0.3382 x2 Format : 0.335 s2 (kJ/kgK) Format : 0.4832 Sgen (kW/K) Format : 0.0504arrow_forward
- Air enters a compressor steadily at the ambient conditions of 100kPa and 20 degrees celcius and leaves at 900 kPa. Heat is lost from the compressor in the amount of 150kJ/kg and the air experiences an entropy decrease of 0.50kJ/kg*K. Using constant specific heats, determine (a) air exit temperature, (b) work input to the compressor, and (c) entropy generation during the process.arrow_forwardRefrigerant 12 is expanded steadily in an isothermal (constant temperature) process. The flow rate is 13.6 kg/min with an inlet state of wet saturated vapor with an 80% quality to a final state of 70°C and 200 kPa. The change of kinetic energy across the device is 3.5 kJ/kg and the heat added is 21.81 kW. Determine the system power.arrow_forwardWater flows through a horizontal coil heated from the outside by high temperature flue gases. As it passes through the coil, the water changes state from liquid at 200 kPa and 80 deg C to vapor at 100kPa and 125 deg C. Its entering velocity is 3m/s and exit velocity is 200 m/s. Determine the heat transferred through the coil per unit mass, Enthalpies of inlet and outlet streams are 334.9 kJ/kg and 2726.5 kJ/kgarrow_forward
- Steam enters the condenser of a steam power plant at 20000 kPa and a quality of 95 percent with a mass flow rate of 20 Mg/h. It is to be cooled by water from a nearby river in circulating the water through the tubes within the condenser. To prevent thermal pollution, the river water is not allowed to experience a temperature rise above 10°C. If the steam is to leave the condenser as saturated liquid at 20000 Pa, determine the mass flow rate of the cooling water required. m = 20,000 kg/h P = 20 kPa = 0.95 %3D Steam Water T+ 10°C P = 20 kPa Sat. liquidarrow_forward2arrow_forwardAn ideal gas expands in an adiabatic turbine from 1200 K and 900 kPa to 800 K. Determine the turbine inlet volume flow rate of the gas, in m3/s, required to produce turbine work output at the rate of 650 kW. The average values of the specific heats for this gas over the temperature range and the gas constant are cp = 1.13 kJ/kg·K, cv = 0.83 kJ/kg·K, and R = 0.30 kJ/kg·K.arrow_forward
- Q: Five hundred kilograms per hour of steam drives a turbine. The steam enters the turbine at 44 atm and 450°C at a linear velocity of 60 m/s and leaves at a point 5 m below the turbine inlet at atmospheric pressure and a velocity of 360 m/s. The turbine delivers shaft work at a rate of 70 kW, and the heat loss from the turbine is estimated to be 10ʻ kcal/h. Calculate the specific enthalpy change associated with the process.arrow_forwardAir enters the compressor of a gas turbine plant at ambient conditions of 100 kPa and 25 ⁰C with a low velocity and exits at 1 MPa and 347 ⁰C with a velocity of 90 m/s. The compression process is adiabatic, and the power input is 271. Determine the mass flowrate of the air through the compressor.arrow_forwardLiquid water enters an adiabatic piping system at 15°C at a rate of 8 kg/s. If the water temperature rises by 0.2°C during flow due to friction, the rate of entropy generation in the pipe is (a) 23 W/K (b) 55 W/K (c) 68 W/K (d) 220 W/K (e) 443 W/Karrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





