
EP CAMPBELL BIO.FOCUS-MOD.MASTER.(18WK)
3rd Edition
ISBN: 9780136781851
Author: Urry
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 9TYU
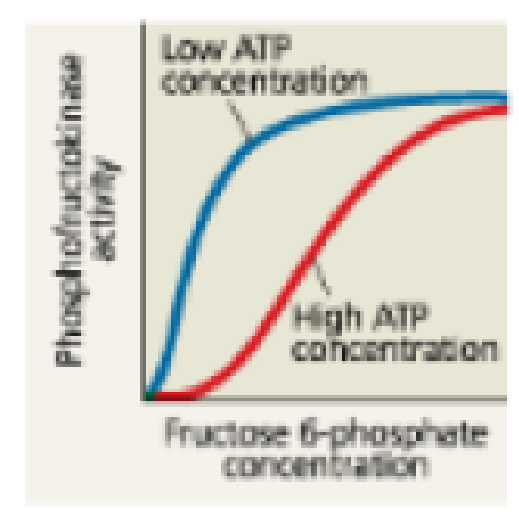
INTERPRET THE DATA Phosphofructokinase is an enzyme that acts on fructose 6-phosphate at an early step in glucose breakdown (step 3 in Figure 7.9). Negative regulation of this enzyme by ATP and positive regulation by AMP control whether the sugar will continue on in the glycolytic pathway. Considering this graph, under which condition Is phosphofructokinase more active? Given this enzyme’s role in glycolysis, explain why it makes sense that ATP and AMP have these effects.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Green Algae, as a group, is actually paraphyletic with one subgroup more closely related to higher plants than the other. Which of the following green algae groups is more closely related to higher plants:
a. Charophyceans
b. Chlorophyceans
c. Rhodophyta
d. Xanthophyceans
A single-celled green algal genus that is motile with 2 flagella, has a cup shaped chloroplast, and an eyespot:
a. Volvox
b. Chlamydomonas
c. Euglena
d. Codium
A[n] ___ is produced by members of the Myxomycota when there is a lack of moisture.
a. plasmodiocarp
b. aethalium
c. sclerotium
d. plasmodium
Chapter 7 Solutions
EP CAMPBELL BIO.FOCUS-MOD.MASTER.(18WK)
Ch. 7.1 - Compare and contrast aerobic and anaerobic...Ch. 7.1 - Name and describe the two ways in which ATP is...Ch. 7.1 - Prob. 3CCCh. 7.2 - During step 6 in Figure 7.9, which molecule acts...Ch. 7.3 - Name the molecules that conserve most of the...Ch. 7.3 - Prob. 2CCCh. 7.4 - Prob. 1CCCh. 7.4 - Prob. 2CCCh. 7.4 - MAKE CONNECTIONS Membranes must be fluid to...Ch. 7.5 - Prob. 1CC
Ch. 7.5 - WHAT IF? A glucose-fed yeast cell is moved from an...Ch. 7.6 - MAKE CONNECTIONS Compare the structure of a fat...Ch. 7.6 - Prob. 2CCCh. 7.6 - WHAT IF? During intense exercise, can a muscle...Ch. 7 - The immediate energy source that drives ATP...Ch. 7 - Which metabolic pathway is common to both...Ch. 7 - In mitochondria, exergonic redox reactions A. are...Ch. 7 - The final electron acceptor of the electron...Ch. 7 - What is the oxidizing agent in the following...Ch. 7 - When electrons flow along the electron transport...Ch. 7 - Most co, from catabolism is released during A....Ch. 7 - DRAW IT The graph here shows the pH difference...Ch. 7 - INTERPRET THE DATA Phosphofructokinase is an...Ch. 7 - Prob. 10TYUCh. 7 - FOCUS ON EVOLUTION ATP synthases are found in the...Ch. 7 - Prob. 12TYUCh. 7 - Prob. 13TYU
Additional Science Textbook Solutions
Find more solutions based on key concepts
True or false? Some trails are considered vestigial because they existed long ago.
Biological Science (6th Edition)
An electric motor has an effective resistance of 32.0 and an inductive reactance of 45.0 when working under l...
Fundamentals of Physics Extended
Single penny tossed 20 times and counting heads and tails: Probability (prediction): _______/20 heads ________/...
Laboratory Manual For Human Anatomy & Physiology
Practice Problem 1.22 Which of the following alkenes can exist as cis-trans isomers? Write their structures. Bu...
Organic Chemistry
How does the removal of hydrogen atoms from nutrient molecules result in a loss of energy from the nutrient mol...
SEELEY'S ANATOMY+PHYSIOLOGY
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Which of the following is not true about the life-cycle of Fucus. a. 8 eggs per oogonium b. 64 sperm per antheridium c. eggs are flagellated d. sperm are flagellatedarrow_forwardGreen Algae, as a group, is actually paraphyletic with one subgroup more closely related to higher plants than the other. Which of the following green algae groups is more closely related to higher plants: a. Charophyceans b. Chlorophyceans c. Rhodophyta d. Xanthophyceansarrow_forwardCertain toxic terpenoids in this group is thought to deter herbivory but may also have some anti-tumor activity? a. green algae b. brown algae c. red algae d. golden algae e. none of thesearrow_forward
- In the cellular slime molds, the most common phase is: a. plasmodium b. pseudoplasmodial c. single cells as myxamoebae d. moundingarrow_forwardWhich of the following descriptive terms does not describe Hydrodictyon? a. colonial b. nonmotile c. 1 large reticulated chloroplast in each cell d. all of these describe Hydrodictyonarrow_forwardWhich of the following does not apply to Chara? a. "stoneworts" b. isogamous c. calcified walls d. apical growth with an axis and branchesarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
 Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning

Human Physiology: From Cells to Systems (MindTap ...
Biology
ISBN:9781285866932
Author:Lauralee Sherwood
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Concepts of Biology
Biology
ISBN:9781938168116
Author:Samantha Fowler, Rebecca Roush, James Wise
Publisher:OpenStax College

Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning


Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Cengage Learning
Anaerobic Respiration; Author: Bozeman Science;https://www.youtube.com/watch?v=cDC29iBxb3w;License: Standard YouTube License, CC-BY