
Basic Chemistry
6th Edition
ISBN: 9780134878119
Author: Timberlake, Karen C. , William
Publisher: Pearson,
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 7CI
For parts a to f, consider the loss of electrons by atoms of the element X, and a gain of electrons by atoms of the element Y. Element X is in Group 2A (2), Period 3, and Y is in Group 7A (17), Period 3. (4.2, 5.4, 5.5, 6.2, 6.3)
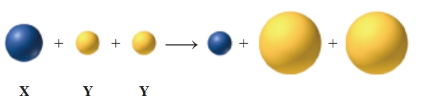
a. Which element is a metal, X or Y?
b. Which element is a nonmetal, X or Y?
c. What are the ionic charges of X and Y?
d. Write the electron configurations for the atoms X and Y.
e. Write the formula and name for the ionic compound formed by the ions of X and Y.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Alcohols can be synthesized using an acid-catalyzed hydration of an alkene. An alkene is combined with aqueous acid (e.. sulfuric acid in water). The
reaction mechanism typically involves a carbocation intermediate.
>
3rd attempt
3343
10
8
Draw arrows to show the reaction between the alkene and hydronium ion.
that
2nd attempt
Feedback
1st attempt
تعمال
Ju See Periodic Table See Hint
F
D
Ju See Periodic Table See Hint
Draw the simplified curved arrow mechanism for the reaction of acetone and CHgLi to give the major product.
4th attempt
Π
Draw the simplified curved arrow mechanism
T
3rd attempt
Feedback
Ju
See Periodic Table See Hint
H
-H
H
-I
H
F
See Periodic Table See Hint
Select the correct reagent to accomplish the first step of this reaction. Then draw a mechanism on the Grignard reagent using curved arrow notation
to show how it is converted to the final product.
4th attempt
Part 1 (0.5 point)
Select the correct reagent to accomplish the first step of this reaction.
Choose one:
OA Mg in ethanol (EtOH)
OB. 2 Li in THF
O C. Li in THF
D. Mg in THF
O E Mg in H2O
Part 2 (0.5 point)
Br
Part 1
Bri
Mg
CH
B
CH,
1 Draw intermediate here, but no arrows.
©
TE
See Periodic Table See Hint
See Hint
ין
H
Chapter 7 Solutions
Basic Chemistry
Ch. 7.1 - What is a mole?Ch. 7.1 - What is Avogadro’s number?Ch. 7.1 - Calculate each of the following: a. number of C...Ch. 7.1 - Calculate each of the following: a. number of Li...Ch. 7.1 - Calculate each of the following quantities in 2.00...Ch. 7.1 - Calculate each of the following quantities in...Ch. 7.1 - Quinine, C20H24N2O2 , is a component of tonic...Ch. 7.1 - Aluminum sulfate, Al2(SO4)3 , is used in some...Ch. 7.1 - Naproxen is used to treat pain and inflammation...Ch. 7.1 - Benadryl is an over-the-counter drug used to treat...
Ch. 7.2 - Calculate the molar mass for each of the...Ch. 7.2 - Calculate the molar mass for each of the...Ch. 7.2 - Calculate the molar mass for each of the...Ch. 7.2 - Calculate the molar mass for each of the...Ch. 7.2 - Calculate the molar mass for each of the...Ch. 7.2 - Calculate the molar mass for each of the...Ch. 7.2 - Calculate the molar mass for each of the...Ch. 7.2 - Calculate the molar mass for each of the...Ch. 7.2 - Calculate the molar mass for each of the...Ch. 7.2 - Calculate the molar mass for each of the...Ch. 7.3 - Calculate the mass, in grams, for each of the...Ch. 7.3 - Calculate the mass, in grams, for each of the...Ch. 7.3 - Calculate the mass, in grams, in 0.150 mol of each...Ch. 7.3 - Calculate the mass, in grams, in 2.28 mol of each...Ch. 7.3 - Calculate the number of moles in each of the...Ch. 7.3 - Calculate the number of moles in each of the...Ch. 7.3 - Calculate the number of moles in 25.0 g of each of...Ch. 7.3 - Calculate the number of moles in 4.00 g of each of...Ch. 7.3 - Calculate the mass, in grams, of C in each of the...Ch. 7.3 - Calculate the mass, in grams, of N in each of the...Ch. 7.3 - Propane gas, C3H8 , is used as a fuel for many...Ch. 7.3 - Allyl sulfide, (C3H5)2S , gives garlic, onions,...Ch. 7.3 - a. The compound MgSO4 , Epsom salts, is used to...Ch. 7.3 - Prob. 34PPCh. 7.3 - Prob. 35PPCh. 7.3 - Prob. 36PPCh. 7.4 - Calculate the mass percent composition of each of...Ch. 7.4 - Calculate the mass percent composition of each of...Ch. 7.4 - Prob. 39PPCh. 7.4 - Prob. 40PPCh. 7.4 - Prob. 41PPCh. 7.4 - Calculate the mass percent of S in each of the...Ch. 7.5 - Calculate the empirical formula for each of the...Ch. 7.5 - Calculate the empirical formula for each of the...Ch. 7.5 - Calculate the empirical formula for each of the...Ch. 7.5 - Calculate the empirical formula for each of the...Ch. 7.6 - Write the empirical formula for each of the...Ch. 7.6 - Prob. 48PPCh. 7.6 - The carbohydrate fructose found in honey and...Ch. 7.6 - Caffeine has an empirical formula of C4H5N2O . If...Ch. 7.6 - Prob. 51PPCh. 7.6 - Glyoxal, used in textiles; maleic acid, used to...Ch. 7.6 - Prob. 53PPCh. 7.6 - Prob. 54PPCh. 7.6 - Vanillic acid contains 57.14% C, 4.80% H, and...Ch. 7.6 - Lactic acid, the substance that builds up in...Ch. 7.6 - A sample of nicotine, a poisonous compound found...Ch. 7.6 - Adenine, a nitrogen-containing compound found in...Ch. 7.6 - Clavulanic acid has a molecular formula of C8H9NO5...Ch. 7.6 - Phenylpropanolamine (PPA) is used to treat urinary...Ch. 7 - The chapter sections to review are shown in...Ch. 7 - The chapter sections to review are shown in...Ch. 7 - The chapter sections to review are shown in...Ch. 7 - Prob. 64UTCCh. 7 - Prob. 65APPCh. 7 - Calculate the mass, in grams, of Cu in each of the...Ch. 7 - Prob. 67APPCh. 7 - Calculate the mass percent composition for each of...Ch. 7 - Prob. 69APPCh. 7 - Prob. 70APPCh. 7 - A mixture contains 0.250 mol of Mn2O3 and 20.0 g...Ch. 7 - A mixture contains 4.001023 molecules of PCl3 and...Ch. 7 - Prob. 73APPCh. 7 - Prob. 74APPCh. 7 - Prob. 75APPCh. 7 - Prob. 76APPCh. 7 - Prob. 77APPCh. 7 - Prob. 78APPCh. 7 - Calculate the molar mass for each of the...Ch. 7 - Calculate the molar mass for each of the...Ch. 7 - Aspirin, C9H8O4 , is used to reduce inflammation...Ch. 7 - Ammonium sulfate, (NH4)2SO4 , is used in...Ch. 7 - Prob. 83APPCh. 7 - Prob. 84APPCh. 7 - Oleic acid, a component of olive oil, is 76.54% C,...Ch. 7 - Prob. 86APPCh. 7 - Prob. 87CPCh. 7 - Prob. 88CPCh. 7 - Prob. 89CPCh. 7 - The following problems are related to the topics...Ch. 7 - For parts a to f, consider the loss of electrons...Ch. 7 - A sterling silver bracelet, which is 92.5% silver...Ch. 7 - Prob. 9CICh. 7 - The active ingredient in an antacid tablet is...Ch. 7 - Oseltamivir, C16H28N2O4 , is a drug that is used...Ch. 7 - Prob. 12CI
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Select the product for the following reaction. HO HO PCC OH ○ OH O HO ○ HO HO HOarrow_forward5:45 Х Select the final product for the following reaction sequence. O O 1. Mg. ether 2.D.Oarrow_forwardBased on the chart Two similarities between the molecule with alpha glycosidic linkages. Two similarities between the molecules with beta glycosidtic linkages. Two differences between the alpha and beta glycosidic linkages.arrow_forward
- please help fill in the tablearrow_forwardAnswer F pleasearrow_forward4. Refer to the data below to answer the following questions: The octapeptide saralasin is a specific antagonist of angiotensin II. A derivative of saralasin is used therapeutically as an antihypertensive. Amino acid analysis of saralasin show the presence of the following amino acids: Ala, Arg, His, Pro, Sar, Tyr, Val, Val A.Sar is the abbreviation for sarcosine, N-methyl aminoethanoic acid. Draw the structure of sarcosine. B. N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward
- What is the structure of the DNA backbone?arrow_forwardPLEASE PLEASE PLEASE use hand drawn structures when possarrow_forward. M 1- MATCH each of the following terms to a structure from the list below. There is only one correct structure for each term and structures may be used more than once. Place the letter of the structure in the blank to the left of the corresponding term. A. Sanger dideoxy method C. Watson-Crick B. GAUCGUAAA D. translation E. HOH2C OH OH G. transcription I. AUGGCUGAG 0 K. OPOH2C 0- OH N- H NH2 F. -OPOH2C 0- OH OH H. Maxam-Gilbert method J. replication N L. HOH2C a. b. C. d. e. f. g. B M. AGATCGCTC a pyrimidine nucleoside RNA base sequence with guanine at the 3' end. DNA base sequence with cytosine at the 3' end. a purine nucleoside DNA sequencing method for the human genome 2'-deoxyadenosine 5'-phosphate process by which mRNA directs protein synthesis OH NH2arrow_forward
- Please use hand drawn structures when neededarrow_forwardB. Classify the following amino acid. Atoms other than carbon and hydrogen are labeled. a. acidic b. basic C. neutral C. Consider the following image. Which level of protein structure is shown here? a. primary b. secondary c. tertiary d. quaternary D. Consider the following image. H RH H HR H R HR HR RH Which level of protein structure is shown in the box? a. primary b. secondary R c. tertiary d. quaternary コー Rarrow_forwardBriefly answer three from the followings: a. What are the four structures of the protein? b. Why is the side chain (R) attached to the alpha carbon in the amino acids is important for the function? c. What are the types of amino acids? And how is it depend on the (R) structure? d. Write a reaction to prepare an amino acid. prodarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY