
7-64 As we shall see in Chapter 20, there are two forms of glucose, designated alpha 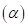 and beta
and beta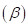 which are in equilibrium in aqueous solution. The equilibrium constant for the reaction is 1.5 at 30°C.
which are in equilibrium in aqueous solution. The equilibrium constant for the reaction is 1.5 at 30°C.
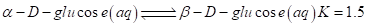
(a) If you begin with a fresh 1.0 M solution of 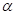 D-glucose in water, what will be its concentration when equilibrium is reached?
D-glucose in water, what will be its concentration when equilibrium is reached?
(b) Calculate the percentage of 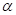 glucose and of
glucose and of 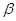 glucose present at equilibrium in aqueous solution at 30°C.
glucose present at equilibrium in aqueous solution at 30°C.
(a)
Interpretation:
The equilibrium concentrations of
Concept Introduction:
Answer to Problem 7.64P
Explanation of Solution
The given equilibrium reaction is,
The initial concentration of
Setting up the equilibrium changes to calculate the equilibrium concentrations:
Therefore,
(b)
Interpretation:
The percentages of
Concept Introduction:
Answer to Problem 7.64P
Percentage of
Percentage of
Explanation of Solution
The given equilibrium reaction is,
The initial concentration of
From the above calculations, concentration of
Percentage of
Percentage of
Want to see more full solutions like this?
Chapter 7 Solutions
Student Solutions Manual for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





