
7-10 The rate of disappearance of HCI was measured for the following reaction:
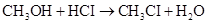
The initial concentration of HCI is 1.85 M. Its concentration decreases to 1.58 M in 54.0 min. What is the
Interpretation:
The rate of given reaction with initial concentration of
Concept introduction:
Rate of reaction: The rate of the reaction is defined as extent of a reaction. For any reaction, it depends on the concentration change of limiting reagent formed in a reaction.
Concentration: Concentration of all chemical species takes part in a reaction depend on their moles present in per liter solution.
The rate is change in concentration of the reactant with change in time. It is mathematically represented as follows:
Answer to Problem 7.10P
Rate of reaction = 0.005 M/min
Explanation of Solution
Given information:
Reaction with initial concentration of
Put the given values in above expression.
Here, negative sign indicates the concentration of HCl decreases.
Rate of reaction = -0.005 M/min
Want to see more full solutions like this?
Chapter 7 Solutions
Student Solutions Manual for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th
- 19.57 Using one of the reactions in this chapter, give the correct starting material (A-L) needed to produce each structure (a-f). Name the type of reaction used. (b) ہ مرد (d) HO (c) དང་ ་་ཡིན་ད་དང་ (f) HO Br B D of oli H J Br K C 人 ↑arrow_forwardInductive effect (+I and -I) in benzene derivatives.arrow_forward7. Helparrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





