
General, Organic, & Biological Chemistry
3rd Edition
ISBN: 9780073511245
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Chapter 7, Problem 7.110P
Interpretation Introduction
Interpretation:
The heating curve is to be drawn when the ice is warmed from
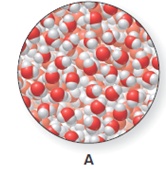
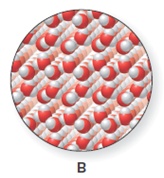
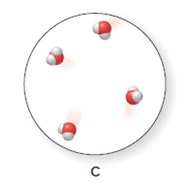
Concept introduction:
The heating curve is a graph which is plotted between the increasing temperature versus the amount of energy absorbed or released by the substances.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
How many grams of solid NaCN have to
be added to 1.5L of water to dissolve 0.18
mol of Fe(OH)3 in the form Fe(CN)63 - ? (
For simplicity, ignore the reaction of CN -
ion with water) Ksp for Fe(OH)3 is 2.8E
-39, and Kform for Fe(CN)63 - is 1.0E31
Draw the most stable chair conformation of 1-ethyl-1-methylcyclohexane, clearly showing the axial and equatorial substituents. [4]
Draw structures corresponding to the following IUPAC name for each of the following compounds; [5]
i) 4-Isopropyl-2,4,5-trimethylheptane
ii) trans-1-tert-butyl-4-ethylcyclohexane
iii) Cyclobutylcycloheptane
iv) cis-1,4-di-isopropylcyclohexane (chair conformation)
v) 3-Ethyl-5-isobutylnonane
Draw and name molecules that meet the following descriptions; [4]
a) An organic molecule containing 2 sp2 hybridised carbon and 1 sp-hybridised carbon atom.
b) A cycloalkene, C7H12, with a tetrasubstituted double bond.
Also answer question 2 from the image
Chapter 7 Solutions
General, Organic, & Biological Chemistry
Ch. 7.1 - Prob. 7.1PCh. 7.2 - Convert each pressure unit to the indicated unit....Ch. 7.3 - Prob. 7.3PCh. 7.3 - Prob. 7.4PCh. 7.3 - Prob. 7.5PCh. 7.3 - Prob. 7.6PCh. 7.3 - Prob. 7.7PCh. 7.3 - Prob. 7.8PCh. 7.3 - The pressure inside a 1.0-L balloon at 25C was 750...Ch. 7.4 - A sample of nitrogen gas contains 5.0 mol in a...
Ch. 7.4 - Prob. 7.11PCh. 7.4 - Prob. 7.12PCh. 7.5 - Prob. 7.13PCh. 7.5 - Prob. 7.14PCh. 7.6 - CO2 was added to a cylinder containing 2.5 atm of...Ch. 7.6 - Prob. 7.16PCh. 7.6 - Prob. 7.17PCh. 7.7 - Prob. 7.18PCh. 7.7 - Prob. 7.19PCh. 7.7 - Prob. 7.20PCh. 7.7 - Which species in each pair has stronger...Ch. 7.7 - Prob. 7.22PCh. 7.7 - Prob. 7.23PCh. 7.8 - Prob. 7.24PCh. 7.8 - Would you predict the surface tension of gasoline,...Ch. 7.9 - Prob. 7.26PCh. 7.10 - Prob. 7.27PCh. 7.10 - The human body is composed of about 70% water. How...Ch. 7.10 - How much energy is required to heat 28.0 g of iron...Ch. 7.10 - Prob. 7.30PCh. 7.10 - Prob. 7.31PCh. 7.10 - If the initial temperature of 120. g of ethanol is...Ch. 7.11 - Use the heat of fusion of water from Sample...Ch. 7.11 - Answer the following questions about water, which...Ch. 7.11 - Prob. 7.35PCh. 7.12 - Answer the following questions about the graph...Ch. 7.12 - How much energy (in calories) is released when...Ch. 7.12 - How much energy (in calories) is required to melt...Ch. 7 - Prob. 7.39PCh. 7 - Prob. 7.40PCh. 7 - Prob. 7.41PCh. 7 - The compressed air tank of a scuba diver reads...Ch. 7 - Assume that each of the following samples is at...Ch. 7 - Use the diagrams in Problem 7.43 to answer the...Ch. 7 - Prob. 7.45PCh. 7 - Prob. 7.46PCh. 7 - Prob. 7.47PCh. 7 - Prob. 7.48PCh. 7 - Prob. 7.49PCh. 7 - Prob. 7.50PCh. 7 - Prob. 7.51PCh. 7 - Prob. 7.52PCh. 7 - Prob. 7.53PCh. 7 - If someone takes a breath and the lungs expand...Ch. 7 - Prob. 7.55PCh. 7 - Prob. 7.56PCh. 7 - Prob. 7.57PCh. 7 - Prob. 7.58PCh. 7 - Prob. 7.59PCh. 7 - Prob. 7.60PCh. 7 - Prob. 7.61PCh. 7 - Prob. 7.62PCh. 7 - Prob. 7.63PCh. 7 - Prob. 7.64PCh. 7 - Prob. 7.65PCh. 7 - Prob. 7.66PCh. 7 - Prob. 7.67PCh. 7 - Prob. 7.68PCh. 7 - Prob. 7.69PCh. 7 - Prob. 7.70PCh. 7 - Prob. 7.71PCh. 7 - Prob. 7.72PCh. 7 - Prob. 7.73PCh. 7 - Prob. 7.74PCh. 7 - Prob. 7.75PCh. 7 - Prob. 7.76PCh. 7 - Prob. 7.77PCh. 7 - Prob. 7.78PCh. 7 - Prob. 7.79PCh. 7 - Prob. 7.80PCh. 7 - Prob. 7.81PCh. 7 - Prob. 7.82PCh. 7 - Prob. 7.83PCh. 7 - Prob. 7.84PCh. 7 - Which molecules are capable of intermolecular...Ch. 7 - Prob. 7.86PCh. 7 - Prob. 7.87PCh. 7 - Explain why the boiling point of A is higher than...Ch. 7 - Prob. 7.89PCh. 7 - Prob. 7.90PCh. 7 - Prob. 7.91PCh. 7 - Prob. 7.92PCh. 7 - Prob. 7.93PCh. 7 - Prob. 7.94PCh. 7 - Prob. 7.95PCh. 7 - Prob. 7.96PCh. 7 - Prob. 7.97PCh. 7 - Prob. 7.98PCh. 7 - Prob. 7.99PCh. 7 - How many calories of heat are needed to increase...Ch. 7 - Prob. 7.101PCh. 7 - If it takes 37.0 cal of heat to raise the...Ch. 7 - Prob. 7.103PCh. 7 - What phase change is shown in the accompanying...Ch. 7 - Prob. 7.105PCh. 7 - Which process requires more energy, melting 250 g...Ch. 7 - Consider the cooling curve drawn below a. Which...Ch. 7 - Prob. 7.108PCh. 7 - Draw the heating curve that is observed when...Ch. 7 - Prob. 7.110PCh. 7 - Use the following values to answer each part. The...Ch. 7 - Prob. 7.112PCh. 7 - If you pack a bag of potato chips for a snack on a...Ch. 7 - Prob. 7.114PCh. 7 - Prob. 7.115PCh. 7 - Prob. 7.116PCh. 7 - Prob. 7.117PCh. 7 - If a scuba diver inhales 0.50 L of air at a depth...Ch. 7 - Prob. 7.119CPCh. 7 - As we learned in Chapter 5, an automobile airbag...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- H 14. Draw the line angle form of the following molecule make sure you use the proper notation to indicate spatial positioning of atoms. F F H 15. Convert the following condensed form to line angle form: (CH3)3CCH2COCH2CON(CH2CH3)2arrow_forwardIn a reaction between two reactants A and B, the half-life is the same for both only if(A) the stoichiometry A:B is 1:1.(B) the stoichiometry A:B is 1:2 or 2:1.arrow_forwardIn a reaction between two reactants A and B, the half-life is the same for both.(1) Only if the stoichiometry A:B is 1:1.(2) If the initial quantities of A and B are in their stoichiometric ratios.arrow_forward
- There are 48 pairs of students in the following table. Each pair has quantitatively determined the mass of taurine in a 250 mL can of the popular energy drink marketed as “Munster” using High Performance Liquid Chromatography (HPLC). The class results are presented below: QUESTION: Calculate the measurement of uncertainty and provide the data in a spreadsheet table. Mass of Taurine (mg) Mass of Taurine (mg) (Table continued) 152.01 152.23 151.87 151.45 154.11 152.64 152.98 153.24 152.88 151.45 153.49 152.48 150.68 152.33 151.52 153.63 152.48 151.68 153.17 153.40 153.77 153.67 152.34 153.16 152.57 153.02 152.86 151.50 151.23 152.57 152.72 151.54 146.47 152.38 152.44 152.54 152.53 152.54 151.32 152.87 151.24 153.26 152.02 152.90 152.87 151.49 152.46 152.58arrow_forward1. Predict the organic product(s) of the following reactions. Assume excess of reagents unless otherwise noted. a) &l BH3 •THF b) 1) NaOH 2) H3O+ solve d) ala 1) EtMgBr 2) H3O+ e) H2N سكر CuLi NH2 1) SOCI2 2) EtMgBr 3) H3O+ NC H3O+ Δarrow_forwardThere are 48 pairs of students in the following table. Each pair has quantitatively determined the mass of taurine in a 250 mL can of the popular energy drink marketed as “Munster” using High Performance Liquid Chromatography (HPLC). The class results are presented below: QUESTION: Summarise and report these results including an indication of measurement uncertainty. In both calculation samples calculate if an outlier is present, max value, number of samples, mean, standard deviation, g (suspect), g (critical) and t (critical). Mass of Taurine (mg) Mass of Taurine (mg) (Table continued) 152.01 152.23 151.87 151.45 154.11 152.64 152.98 153.24 152.88 151.45 153.49 152.48 150.68 152.33 151.52 153.63 152.48 151.68 153.17 153.40 153.77 153.67 152.34 153.16 152.57 153.02 152.86 151.50 151.23 152.57 152.72 151.54 146.47 152.38 152.44 152.54 152.53 152.54 151.32…arrow_forward
- Indicate the rate expressions for reactions that have order 0, 1, and 2.arrow_forwardPROBLEMS Q1) Label the following salts as either acidic, basic, or neutral a) Fe(NOx) c) AlBr b) NH.CH COO d) HCOON (1/2 mark each) e) Fes f) NaBr Q2) What is the pH of a 0.0750 M solution of sulphuric acid?arrow_forward8. Draw all the resonance forms for each of the fling molecules or ions, and indicate the major contributor in each case, or if they are equivalent (45) (2) -PH2 سمة مدarrow_forward
- A J то گای ه +0 Also calculate the amount of starting materials chlorobenzaldehyde and p-chloroacetophenone required to prepare 400 mg of the given chalcone product 1, 3-bis(4-chlorophenyl)prop-2-en-1-one molar mass ok 1,3-bis(4-Chlorophenyl) prop-2-en-1-one = 277.1591m01 number of moles= 0.400/277.15 = 0.00144 moles 2 x 0.00 144=0.00288 moves arams of acetophenone = 0.00144 X 120.16 = 0.1739 0.1739x2=0.3469 grams of benzaldehyde = 0.00144X106.12=0.1539 0.1539x2 = 0.3069 Starting materials: 0.3469 Ox acetophenone, 0.3069 of benzaldehyde 3arrow_forward1. Answer the questions about the following reaction: (a) Draw in the arrows that can be used make this reaction occur and draw in the product of substitution in this reaction. Be sure to include any relevant stereochemistry in the product structure. + SK F Br + (b) In which solvent would this reaction proceed the fastest (Circle one) Methanol Acetone (c) Imagine that you are working for a chemical company and it was your job to perform a similar reaction to the one above, with the exception of the S atom in this reaction being replaced by an O atom. During the reaction, you observe the formation of three separate molecules instead of the single molecule obtained above. What is the likeliest other products that are formed? Draw them in the box provided.arrow_forward3. For the reactions below, draw the arrows corresponding to the transformations and draw in the boxes the reactants or products as indicated. Note: Part A should have arrows drawn going from the reactants to the middle structure and the arrows on the middle structure that would yield the final structure. For part B, you will need to draw in the reactant before being able to draw the arrows corresponding to product formation. A. B. Rearrangement ΘΗarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
GCSE Chemistry - Differences Between Compounds, Molecules & Mixtures #3; Author: Cognito;https://www.youtube.com/watch?v=jBDr0mHyc5M;License: Standard YouTube License, CC-BY