
General, Organic, & Biological Chemistry
3rd Edition
ISBN: 9780073511245
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 7, Problem 7.88P
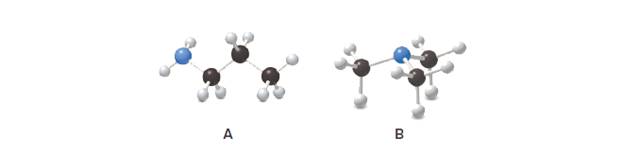
Explain why the boiling point of A is higher than the boiling point of B despite thefact that A and B have the same chemical formula
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
General, Organic, & Biological Chemistry
Ch. 7.1 - Prob. 7.1PCh. 7.2 - Convert each pressure unit to the indicated unit....Ch. 7.3 - Prob. 7.3PCh. 7.3 - Prob. 7.4PCh. 7.3 - Prob. 7.5PCh. 7.3 - Prob. 7.6PCh. 7.3 - Prob. 7.7PCh. 7.3 - Prob. 7.8PCh. 7.3 - The pressure inside a 1.0-L balloon at 25C was 750...Ch. 7.4 - A sample of nitrogen gas contains 5.0 mol in a...
Ch. 7.4 - Prob. 7.11PCh. 7.4 - Prob. 7.12PCh. 7.5 - Prob. 7.13PCh. 7.5 - Prob. 7.14PCh. 7.6 - CO2 was added to a cylinder containing 2.5 atm of...Ch. 7.6 - Prob. 7.16PCh. 7.6 - Prob. 7.17PCh. 7.7 - Prob. 7.18PCh. 7.7 - Prob. 7.19PCh. 7.7 - Prob. 7.20PCh. 7.7 - Which species in each pair has stronger...Ch. 7.7 - Prob. 7.22PCh. 7.7 - Prob. 7.23PCh. 7.8 - Prob. 7.24PCh. 7.8 - Would you predict the surface tension of gasoline,...Ch. 7.9 - Prob. 7.26PCh. 7.10 - Prob. 7.27PCh. 7.10 - The human body is composed of about 70% water. How...Ch. 7.10 - How much energy is required to heat 28.0 g of iron...Ch. 7.10 - Prob. 7.30PCh. 7.10 - Prob. 7.31PCh. 7.10 - If the initial temperature of 120. g of ethanol is...Ch. 7.11 - Use the heat of fusion of water from Sample...Ch. 7.11 - Answer the following questions about water, which...Ch. 7.11 - Prob. 7.35PCh. 7.12 - Answer the following questions about the graph...Ch. 7.12 - How much energy (in calories) is released when...Ch. 7.12 - How much energy (in calories) is required to melt...Ch. 7 - Prob. 7.39PCh. 7 - Prob. 7.40PCh. 7 - Prob. 7.41PCh. 7 - The compressed air tank of a scuba diver reads...Ch. 7 - Assume that each of the following samples is at...Ch. 7 - Use the diagrams in Problem 7.43 to answer the...Ch. 7 - Prob. 7.45PCh. 7 - Prob. 7.46PCh. 7 - Prob. 7.47PCh. 7 - Prob. 7.48PCh. 7 - Prob. 7.49PCh. 7 - Prob. 7.50PCh. 7 - Prob. 7.51PCh. 7 - Prob. 7.52PCh. 7 - Prob. 7.53PCh. 7 - If someone takes a breath and the lungs expand...Ch. 7 - Prob. 7.55PCh. 7 - Prob. 7.56PCh. 7 - Prob. 7.57PCh. 7 - Prob. 7.58PCh. 7 - Prob. 7.59PCh. 7 - Prob. 7.60PCh. 7 - Prob. 7.61PCh. 7 - Prob. 7.62PCh. 7 - Prob. 7.63PCh. 7 - Prob. 7.64PCh. 7 - Prob. 7.65PCh. 7 - Prob. 7.66PCh. 7 - Prob. 7.67PCh. 7 - Prob. 7.68PCh. 7 - Prob. 7.69PCh. 7 - Prob. 7.70PCh. 7 - Prob. 7.71PCh. 7 - Prob. 7.72PCh. 7 - Prob. 7.73PCh. 7 - Prob. 7.74PCh. 7 - Prob. 7.75PCh. 7 - Prob. 7.76PCh. 7 - Prob. 7.77PCh. 7 - Prob. 7.78PCh. 7 - Prob. 7.79PCh. 7 - Prob. 7.80PCh. 7 - Prob. 7.81PCh. 7 - Prob. 7.82PCh. 7 - Prob. 7.83PCh. 7 - Prob. 7.84PCh. 7 - Which molecules are capable of intermolecular...Ch. 7 - Prob. 7.86PCh. 7 - Prob. 7.87PCh. 7 - Explain why the boiling point of A is higher than...Ch. 7 - Prob. 7.89PCh. 7 - Prob. 7.90PCh. 7 - Prob. 7.91PCh. 7 - Prob. 7.92PCh. 7 - Prob. 7.93PCh. 7 - Prob. 7.94PCh. 7 - Prob. 7.95PCh. 7 - Prob. 7.96PCh. 7 - Prob. 7.97PCh. 7 - Prob. 7.98PCh. 7 - Prob. 7.99PCh. 7 - How many calories of heat are needed to increase...Ch. 7 - Prob. 7.101PCh. 7 - If it takes 37.0 cal of heat to raise the...Ch. 7 - Prob. 7.103PCh. 7 - What phase change is shown in the accompanying...Ch. 7 - Prob. 7.105PCh. 7 - Which process requires more energy, melting 250 g...Ch. 7 - Consider the cooling curve drawn below a. Which...Ch. 7 - Prob. 7.108PCh. 7 - Draw the heating curve that is observed when...Ch. 7 - Prob. 7.110PCh. 7 - Use the following values to answer each part. The...Ch. 7 - Prob. 7.112PCh. 7 - If you pack a bag of potato chips for a snack on a...Ch. 7 - Prob. 7.114PCh. 7 - Prob. 7.115PCh. 7 - Prob. 7.116PCh. 7 - Prob. 7.117PCh. 7 - If a scuba diver inhales 0.50 L of air at a depth...Ch. 7 - Prob. 7.119CPCh. 7 - As we learned in Chapter 5, an automobile airbag...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
On what molecule does the anticodon appear? Explain the role of this molecule in protein synthesis.
Human Physiology: An Integrated Approach (8th Edition)
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In each of the following groups of substances, pick the one that has the given property. Justify your answer. a. highest boiling point: HBr, Kr, or Cl2 b. highest freezing point: H2O, NaCl, or HF c. lowest vapor pressure at 25C: Cl2, Br2, or I2 d. lowest freezing point: N2, CO, or CO2 e. lowest boiling point: CH4, CH3CH3, or CH3CH2CH3 f. highest boiling point: HF, HCl, or HBr g.arrow_forwardDefine the following and give an example of each: (a) dispersion force (b) dipole-dipole attraction (c) hydrogen bondarrow_forwardUse Figure 11.7 to estimate the boiling point of diethyl ether, (C2H5)2O, under an external pressure of 470 mmHg.arrow_forward
- 5-86 Using the phase diagram of water (Figure 5-20), describe the process by which you can sublime 1 g of ice at-10°C and at 1 atm pressure to water vapor at the same temperature.arrow_forwardOn the basis of intermolecular attractions, explain the differences in the boiling points of n butane (1 C) and chloroethane (12 C), which have similar molar masses.arrow_forwardDiethyl ether (CH3CH2OCH2CH3) was one of the first chemicals used as an anesthetic. At 34.6C, diethyl ether has a vapor pressure of 760. torr, and at 17.9C, it has a vapor pressure of 400. torr. What is the H of vaporization for diethyl ether?arrow_forward
- Suppose that three unknown pure substances are liquids at room temperature. You make vapor pressure measurements and find that substance Q has a pressure of torr, substance R has a pressure of 42 torr, and substance S has a pressure of 330 torr. If you slowly increase the temperature, which substance will boil first and which will boil last?arrow_forwardWhat are intermolecular forces? How do they differ from intramolecular forces? What are dipole-dipole forces? How do typical dipole-dipole forces differ from hydrogen bonding interactions? In what ways are they similar? What are London dispersion forces? How do typical London dispersion forces differ from dipole-dipole forces? In what ways are they similar? Describe the relationship between molecular size and strength of London dispersion forces. Place the major types of intermolecular forces in order of increasing strength. Is there some overlap? That is, can the strongest London dispersion forces be greater than some dipole-dipole forces? Give an example of such an instance.arrow_forwardFor each of the following pairs, choose the member with the lower boiling point. Explain your reason in each case. (a) NaCl or PCl3 (b) NH3 or AsH3 (c) C3H7OH or C2H5OCH3 (d) HI(g) or HCl(g)arrow_forward
- Of the four general types of solids, which one(s) (a) are generally low-boiling? (b) are ductile and malleable? (c) are generally soluble in nonpolar solvents?arrow_forward8.51 Suppose that three unknown pure substances are liquids at room temperature. You determine that the boiling point of substance A is 53°C, that of substance B is 117°C, and that of substance C is 77°C. Based on this information, rank the three substances in order of their vapor pressures at room temperature.arrow_forwardChloroform, CHCl3, has a normal boiling point of 61C. Its vapor pressure at 43C is 0.526 atm. What is the concentration (in g/L) of CHCl3 when it saturates the air at 27C?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY