
Concept explainers
(a)
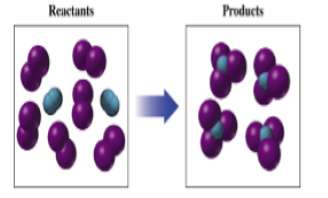
Interpretation: To write the chemical formula for each reactants and products given in a
Concept Introduction:
Mass can neither be created nor be destroyed.
A chemical reaction should be well balance i.e. the number of atoms of each element in reactant side must be equal to number of atoms in product side.
According to the question:
Purple sphere represents Iodine-atom (I).
Blue sphere represents Nitrogen-atom (N).
There are two reactants and one product as follows:
Reactants = Iodine molecule (I2) and nitrogen molecule (N2).
Products = Nitrogen triiodide (HI).
According to the question:
Purple sphere = Iodine -atom
Blue sphere = Nitrogen-atom
Therefore,
The first reactant is:
 = I2known as iodine molecule.
= I2known as iodine molecule.
The second reactant is:
 = N2known as nitrogen molecule or dinitrogen.
= N2known as nitrogen molecule or dinitrogen.
The product is:
 = NI3known as nitrogen triiodide.
= NI3known as nitrogen triiodide.
(b)
Interpretation: To balance the given chemical reaction:
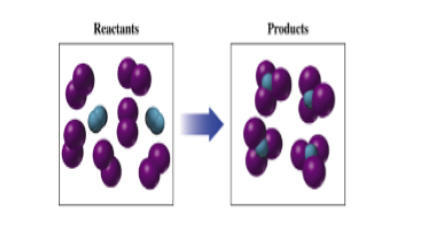
Concept Introduction:
Mass can neither be created nor be destroyed.
A chemical reaction should be well balance i.e. the number of atoms of each element in reactant side must be equal to number of atoms in product side.
Purple sphere = Iodine atom
Blue sphere = Nitrogen atom
(c)
Interpretation: To indicate the type of given chemical reaction:
Concept Introduction:
Types of chemical reaction:
- Combination reaction: Here two or more reactants combines to form a single product
- Decomposition product: In this type of reaction, one reactant is breakdown into two or more products
- Displacement reaction: This is a type of reaction where more reactive element displaces the less reactive element.
- Double-displacement reaction: In this type of reaction, two compounds react in such a way that cations and anions of two reactants interchange their place.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
- help 20arrow_forwardProvide the drawing of the unknown structure that corresponds with this data.arrow_forward20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forward
- 3) Draw a detailed mechanism and predict the product of the reaction shown? 1) EtMgBr 2) H3O+arrow_forwardHow to draw the mechanism for this reaction?arrow_forward> H₂C=C-CH2-CH3 B. H₂O Pt C. + H2 + H₂O H D. 16. Give the IUPAC name for each of the following: B. Cl Cl c. Cl Cl 17. Draw the line-angle formula for each of the following compounds: 1. phenol 2. 1,3-dichlorobenzene 3. 4-ethyltoluene < Previous Submit Assignment Next ▸arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





