
EBK INTRO.CHEMISTRY (NASTA EDITION)
9th Edition
ISBN: 9781337678032
Author: ZUMDAHL
Publisher: CENGAGE CO
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 7, Problem 39QAP
What salt would form when each of the following strong acid/ strong base reactions takes place?
l type='a'>
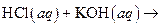
i>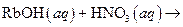
i>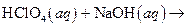
i>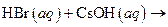
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Bookmarks
Profiles Tab Window Help
Chemical Formula - Aktiv Che X
+
→ C
11
a
app.aktiv.com
Google Chrome isn't your default browser Set as default
Question 12 of 16
Q Fri Feb 2
Verify it's you
New Chrome availabl-
Write the balanced molecular chemical equation for the reaction in aqueous solution for
mercury(I) nitrate and chromium(VI) sulfate. If no reaction occurs, simply write only NR. Be
sure to include the proper phases for all species within the reaction.
3 Hg(NO3)2(aq) + Cг2(SO4)3(aq) → 3 Hg₂SO (s) + 2 Cr(NO3), (aq)
ean Ui
mate co
ence an
climate
bility inc
ulnerabili
women,
main critic
CLIMATE-INI
ernational
+
10
O
2
W
FEB
1
+
4-
3-
2-
2
2
(
3
4
NS
28
2
ty
56
+
2+
3+
4+
7
8
9 0
5
(s)
(1)
Ch
O
8
9
(g) (aq)
Hg
NR
CI
Cr
x H₂O
A
80
Q
A
DII
A
F2
F3
FA
F5
F6
F7
F8
F9
#3
EA
$
do 50
%
6
CO
&
7
E
R
T
Y
U
8
(
9
0
F10
34
F11
川
F12
Subr
+
delete
0
{
P
}
Deducing the reactants of a Diels-Alder reaction
n the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one
step, by moderately heating the reactants?
?
Δ
• If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any
arrangement you like.
• If your answer is no, check the box under the drawing area instead.
Explanation Check
Click and drag to start drawing a structure.
>
Predict the major products of the following organic reaction:
+
Some important notes:
A
?
• Draw the major product, or products, of the reaction in the drawing area below.
• If there aren't any products, because no reaction will take place, check the box below the drawing area instead.
• Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are
enantiomers.
Explanation
Check
Click and drag to start drawing a structure.
Chapter 7 Solutions
EBK INTRO.CHEMISTRY (NASTA EDITION)
Ch. 7.2 - at if no ionic solids were soluble in water? Could...Ch. 7.2 - Prob. 7.1SCCh. 7.3 - trong>Exercise 7.2 For each of the following...Ch. 7.5 - Prob. 7.3SCCh. 7.7 - lton believed that atoms were indivisible. Thomson...Ch. 7.7 - ercise 7.4 Classify each of the following...Ch. 7 - onsider the mixing of aqueous solutions of...Ch. 7 - ssume a highly magnified view of a solution of HCI...Ch. 7 - hy is the formation of a solid evidence of a...Ch. 7 - Prob. 4ALQ
Ch. 7 - ixing an aqueous solution of potassium nitrate...Ch. 7 - Prob. 6ALQCh. 7 - se the Arrhenius definition of acids and bases to...Ch. 7 - Prob. 8ALQCh. 7 - hy is the formation of a gas evidence of a...Ch. 7 - Label each of the following statements as true or...Ch. 7 - Look at Fig. 7.2 in the text. It is possible for a...Ch. 7 - What is the purpose of spectator ions? If they are...Ch. 7 - Which of the following must be an...Ch. 7 - If an element is a reactant or product in a...Ch. 7 - Prob. 15ALQCh. 7 - On the basis of the general solubility rules given...Ch. 7 - Write the balanced formula and net ionic equation...Ch. 7 - hy is water an important solvent? Although you...Ch. 7 - hat is a “driving force”? What are some of the...Ch. 7 - Prob. 3QAPCh. 7 - Prob. 4QAPCh. 7 - escribe briefly what happens when an ionic...Ch. 7 - hen the ionic solute K3PO4is dissolved in water,...Ch. 7 - Prob. 7QAPCh. 7 - ow do chemists know that the ions behave...Ch. 7 - uppose you are trying to help your friend...Ch. 7 - Using the general solubility rules given in Table...Ch. 7 - On the basis of the general solubility rules given...Ch. 7 - On the basis of the general solubility rules given...Ch. 7 - On the basis of the general solubility rules given...Ch. 7 - On the basis of the general solubility rules given...Ch. 7 - On the basis of the general solubility rules given...Ch. 7 - Lead(II) nitrate is added to four separate beakers...Ch. 7 - On the basis of the general solubility rules given...Ch. 7 - On the basis of the general solubility rules given...Ch. 7 - Balance each of the following equations that...Ch. 7 - Balance each of the following equations that...Ch. 7 - For each of the following precipitation reactions,...Ch. 7 - A solution of zinc nitrate is mixed with a...Ch. 7 - What is a net ionic equation? What species are...Ch. 7 - Prob. 24QAPCh. 7 - Based on the general solubility rules given in...Ch. 7 - Write the balanced molecular, complete ionic, and...Ch. 7 - Many chromate salts are insoluble, and most have...Ch. 7 - The procedures and principles of qualitative...Ch. 7 - Many plants are poisonous because their stems and...Ch. 7 - Prob. 30QAPCh. 7 - What is meant by a strong acid? Are the strong...Ch. 7 - What is meant by a strong base? Are the strong...Ch. 7 - The same net ionic process takes place when any...Ch. 7 - Prob. 34QAPCh. 7 - If 1000 NaOH units were dissolved in a sample of...Ch. 7 - What is a salt? Give two balanced chemical...Ch. 7 - Write balanced equations showing how three of the...Ch. 7 - Prob. 38QAPCh. 7 - What salt would form when each of the following...Ch. 7 - Prob. 40QAPCh. 7 - Prob. 41QAPCh. 7 - Give an example of a simple chemical reaction that...Ch. 7 - What do we mean when we say that the transfer of...Ch. 7 - Prob. 44QAPCh. 7 - If atoms of the metal calcium were to react with...Ch. 7 - If oxygen molecules, were to react with magnesium...Ch. 7 - Prob. 47QAPCh. 7 - Prob. 48QAPCh. 7 - Prob. 49QAPCh. 7 - Prob. 50QAPCh. 7 - Prob. 51QAPCh. 7 - The reaction between ammonium perchlorate and...Ch. 7 - Identify each of the following unbalanced reaction...Ch. 7 - Prob. 54QAPCh. 7 - Prob. 55QAPCh. 7 - Prob. 56QAPCh. 7 - What is a synthesis or combination reaction? Give...Ch. 7 - What is a decomposition reaction? Give an example....Ch. 7 - Complete and balance each of the following...Ch. 7 - Prob. 60QAPCh. 7 - Prob. 61QAPCh. 7 - Prob. 62QAPCh. 7 - Balance each of the following equations that...Ch. 7 - Prob. 64QAPCh. 7 - Balance each of the following equations that...Ch. 7 - Prob. 66QAPCh. 7 - Distinguish between the molecular equation, the...Ch. 7 - Prob. 68APCh. 7 - Without first writing a full molecular or ionic...Ch. 7 - Complete and balance each of the following...Ch. 7 - Prob. 71APCh. 7 - Prob. 72APCh. 7 - Prob. 73APCh. 7 - Prob. 74APCh. 7 - For each of the following unbalanced molecular...Ch. 7 - Write the balanced molecular, complete ionic, and...Ch. 7 - What strong acid and what strong base would react...Ch. 7 - Prob. 78APCh. 7 - For the reaction 16Fe(s)+3S8(s)8Fe2S3(s), show how...Ch. 7 - Prob. 80APCh. 7 - Identify each of the following unbalanced reaction...Ch. 7 - Which of the following statements is/are true...Ch. 7 - Prob. 83APCh. 7 - Prob. 84APCh. 7 - Prob. 85APCh. 7 - Prob. 86APCh. 7 - Prob. 87APCh. 7 - For each of the following metals, how many...Ch. 7 - For each of the following nonmetals, how many...Ch. 7 - True or false? When solutions of barium hydroxide...Ch. 7 - Classify the reactions represented by the...Ch. 7 - When a sodium chromate solution and aluminum...Ch. 7 - Prob. 93APCh. 7 - Consider a solution with the following ions...Ch. 7 - Prob. 95APCh. 7 - For the following chemical reactions, determine...Ch. 7 - Prob. 97CPCh. 7 - hat kind of visual evidence indicates that a...Ch. 7 - Prob. 2CRCh. 7 - Prob. 3CRCh. 7 - Prob. 4CRCh. 7 - hat is meant by the driving force for a reaction?...Ch. 7 - xplain to your friend what chemists mean by a...Ch. 7 - efine the term strong electrolyte. What types of...Ch. 7 - ummarize the simple solubility rules for ionic...Ch. 7 - n general terms, what are the spectator ions in a...Ch. 7 - Describe some physical and chemical properties of...Ch. 7 - Prob. 11CRCh. 7 - What do we call reactions in which electrons are...Ch. 7 - What is a combustion reaction? Are combustion...Ch. 7 - Prob. 14CRCh. 7 - List and define all the ways of classifying...Ch. 7 - The element carbon undergoes many inorganic...Ch. 7 - Prob. 17CRCh. 7 - The reagent shelf in a general chemistry lab...Ch. 7 - Prob. 19CRCh. 7 - Prob. 20CRCh. 7 - Prob. 21CRCh. 7 - Prob. 22CRCh. 7 - Using the general solubility rules discussed in...Ch. 7 - Write the balanced net ionic equation for the...Ch. 7 - Prob. 25CR
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- if the answer is no reaction than state that and please hand draw!arrow_forward"I have written solutions in text form, but I need experts to rewrite them in handwriting from A to Z, exactly as I have written, without any changes."arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Please correct answer and don't used hand raitingarrow_forwardreciprocal lattices rotates along with the real space lattices of the crystal. true or false?arrow_forwardDeducing the reactants of a Diels-Alder reaction vn the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ O If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Click and drag to start drawing a structure. Product can't be made in one step. Explanation Checkarrow_forward
- Predict the major products of the following organic reaction: Δ ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. Larrow_forward> Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accesarrow_forwardPredict the major products of the following organic reaction: O O + A ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. eserved. Terms of Use | Privacy Center >arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
Thermogravimetric Analysis [ TGA ] # Thermal Analysis # Analytical Chemistry Part-11# CSIR NET/GATE; Author: Priyanka Jain;https://www.youtube.com/watch?v=p1K-Jpzylso;License: Standard YouTube License, CC-BY