
Concept explainers
(a)
Interpretation:
The
Concept Introduction:
Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
(a)
Explanation of Solution
Given molecule is
The Lewis electron dot structure for
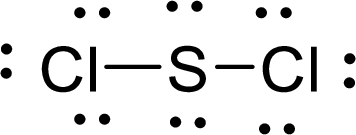 .
.
The electron-region geometry of Sulphur atom bonded to two other atoms and two lone pair of electron is tetrahedral. It is a type of
(b)
Interpretation:
The
Concept Introduction:
Refer to (a).
(b)
Explanation of Solution
Given molecule is
The Lewis electron dot structure for
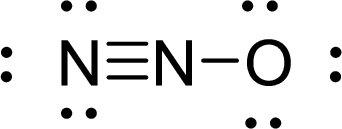 .
.
The electron-region geometry of central atom bonded to two other atoms is linear. It is a type of
(c)
Interpretation:
The bond angles of
Concept Introduction:
Refer to (a).
(c)
Explanation of Solution
Given molecule is
The Lewis electron dot structure for
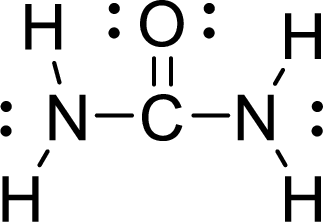 .
.
The electron-region geometry of Carbon atom bonded to three other atoms is triangular planar. It is a type of
The electron-region geometry of second Nitrogen atom bonded to three other atoms and one lone pair of electron is tetrahedral. It is a type of
(d)
Interpretation:
The bond angles of
Concept Introduction:
Refer to (a).
(d)
Explanation of Solution
Given molecule is
The Lewis electron dot structure for
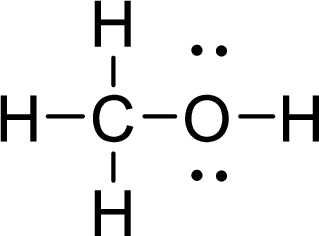 .
.
The electron-region geometry of carbon atom bonded to four other atoms is tetrahedral. It is a type of
The electron-region geometry of oxygen atom bonded to two other atoms and two lone pair of electron tetrahedral. It is a type of
Want to see more full solutions like this?
Chapter 7 Solutions
Bundle: Chemistry: The Molecular Science, 5th, Loose-Leaf + OWLv2 with Quick Prep 24-Months Printed Access Card
- Indicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forward
- Synthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forward
- Indicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
