
Concept explainers
a)
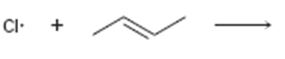
Interpretation:
Curved arrows are to be drawn to show the flow of electrons in the given reaction. The structure of carbon radical that is formed when the halogen radical add to the
Concept introduction:
In reactions involving free radicals homolytic cleavage of covalent bonds takes place. The free radicals produced in the initiation step reacts with the other reactant present in the propagation steps to yield new radicals.
To draw:
Curved arrows to show the flow of electrons in the given reaction and to show the structure of carbon radical that is formed when the halogen radical add to the alkenes.
b)
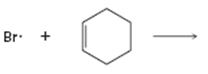
Interpretation:
Curved arrows are to be drawn to show the flow of electrons in the given reaction. The structure of carbon radical that is formed when the halogen radical add to the alkenes is to be drawn.
Concept introduction:
In reactions involving free radicals homolytic cleavage of covalent bonds takes place. The free radicals produced in the initiation step reacts with the other reactant present in the propagation steps to yield new radicals.
To draw:
Curved arrows to show the flow of electrons in the given reaction and to show the structure of carbon radical that is formed when the halogen radical add to the alkenes.
c)
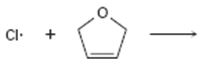
Interpretation:
Curved arrows are to be drawn to show the flow of electrons in the given reaction. The structure of carbon radical that is formed when the halogen radical add to the alkenes is to be drawn.
Concept introduction:
In reactions involving free radicals homolytic cleavage of covalent bonds takes place. The free radicals produced in the initiation step reacts with the other reactant present in the propagation steps to yield new radicals.
To draw:
Curved arrows to show the flow of electrons in the given reaction and to show the structure of carbon radical that is formed when the halogen radical add to the alkenes.
Trending nowThis is a popular solution!

Chapter 6 Solutions
Student Value Bundle: Organic Chemistry, + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card (NEW!!)
- When N,N-dimethylaniline is treated with bromine both the ortho and para products are observed. However when treated with a mixture of nitric acid and sulfuric acid only the meta product is observed. Explain these results and support your answer with the appropriate drawings *Hint amines are bases* N HNO3 H2SO4 N NO2 N Br2 N Br + N 8-8-8 FeBr3 Brarrow_forwardDraw a mechanism that explains the formation of compound OMe SO3H 1. Fuming H2SO4arrow_forwardConsider the following two acid-base reactions: OH OHI Based on what you know about the compounds and their acidity, which direction would you expect both of these reactions to proceed? Show your reasoning. A pKa table has been provided in case you need it. Functional group Example pka CHA -50 Alkane -35 Amine : NH3 Alkyne RH 25 Water HO-H 169 16 10 Protonated amines NH 10 5 Carboxylic acids OH Hydrochloric acid HCI A chemist intends to run the following reaction on the three substrates shown below: H₂O R-CI product room temp. Cl Cl (1) (2) (3) They find one will react quickly, one slowly, and one will not react at all. Which is which, and why? HINT: What is the reaction they're trying to do? Does that mechanism tell you anything about why something would be favored?arrow_forward
- NH3 decomposes through an equilibrium reaction between NH3, H2, and N2. Only one of the options is correct:(A). The mechanism of the NH3 decomposition reaction must necessarily involve the collision of two NH3 molecules to induce a rearrangement of the atoms in this molecule.(B). The molecular weight of the NH3 decomposition reaction is 2 since two NH3 molecules must collide.(C). The rate of the NH3 decomposition reaction must be greater than that of NH3 synthesis, since the former requires two molecules to collide and the latter, four.(D). The NH3 decomposition reaction cannot occur in a single step.arrow_forwardGiven the equilibrium A2 + B2 ⇌ 2 AB where k1 is the rate coefficient of the forward reaction and k-1 is the rate coefficient of the reverse reaction, with the forward reaction being first-order in A2 and B2, and the reverse reaction being second-order in AB. Equilibrium will be reached later if the relative values of the constants are:(A) k1 high and k-1 high(B) k1 high and k-1 low(C) k1 low and k-1 high(D) k1 low and k-1 lowarrow_forwardA 2-step reaction has the following mechanism: | 1. (fast) R2 R+R 2. (slow) R+Q K₂ P k_1 What series does it have? (A). v= - = (k + k1 − k-1)[R2][Q] (B). v=-k₁[R₂] + k₁[R]² - k₂[R][Q] (C). v=k₂[R]²[Q]² (D). v = k[R₂]1/2[Q]arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward

 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





