
Organic Chemistry Plus Mastering Chemistry with Pearson eText -- Access Card Package (9th Edition) (New in Organic Chemistry)
9th Edition
ISBN: 9780321971128
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6.13B, Problem 6.24P
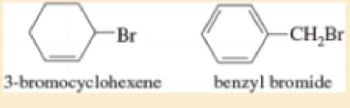
3-Bromocyclohexene is a secondary halide, and benzyl bromide is a primary halide Both halides undergo SN1 substitution about as fast as most tertiary halides Use resonance structures to explain this enhanced reactivity.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
=Naming benzene derivatives
Name these organic compounds:
structure
C1
CH3
name
☐
CH3
ப
C1
×
☐
Blocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see image **NOTE: The compound on the left is the starting point, and the compound on the right is the final product. Please show the steps in between to get from start to final, please. These are not two different compounds that need to be worked.
I dont understand this.
Chapter 6 Solutions
Organic Chemistry Plus Mastering Chemistry with Pearson eText -- Access Card Package (9th Edition) (New in Organic Chemistry)
Ch. 6.1 - Classify each compound as an alkyl halide, a vinyl...Ch. 6.2 - Give the structures of the following compounds. a....Ch. 6.2 - For each of the following compounds, A. give the...Ch. 6.3E - Prob. 6.4PCh. 6.4 - Prob. 6.5PCh. 6.5A - For each pair of compounds, predict which compound...Ch. 6.5B - Prob. 6.7PCh. 6.6B - Prob. 6.8PCh. 6.6B - The light-initiated reaction of...Ch. 6.6B - Show how free-radical halogenation might be used...
Ch. 6.7 - Prob. 6.11PCh. 6.7 - Prob. 6.12PCh. 6.8 - Prob. 6.13PCh. 6.9 - Predict the major products of the following...Ch. 6.9 - Prob. 6.15PCh. 6.10A - Prob. 6.16PCh. 6.11A - When diethyl ether (CH3CH2OCH2CH3) is treated with...Ch. 6.11B - Prob. 6.18PCh. 6.11B - For each pair of compounds, state which compound...Ch. 6.12 - Prob. 6.20PCh. 6.12 - Under appropriate conditions...Ch. 6.13 - Propose an SN1 mechanism for the solvolysis of...Ch. 6.13B - Prob. 6.23PCh. 6.13B - 3-Bromocyclohexene is a secondary halide, and...Ch. 6.15 - Prob. 6.25PCh. 6.15 - Prob. 6.26PCh. 6.16 - For each reaction, give the expected substitution...Ch. 6.16 - Prob. 6.28PCh. 6.16 - Prob. 6.29PCh. 6 - Prob. 6.30SPCh. 6 - Draw the structures of the following compounds. a....Ch. 6 - Give systematic (IUPAC) names for the following...Ch. 6 - Prob. 6.33SPCh. 6 - Predict the compound in each pair that will...Ch. 6 - Prob. 6.35SPCh. 6 - Give two syntheses for (CH3)2CHOCH2CH3, and...Ch. 6 - Prob. 6.37SPCh. 6 - Prob. 6.38SPCh. 6 - Chlorocyclohexane reacts with sodium cyanide...Ch. 6 - Give the substitution products expected from...Ch. 6 - Prob. 6.41SPCh. 6 - Prob. 6.42SPCh. 6 - Two of the carbocations in Problem6-42 are prone...Ch. 6 - Prob. 6.44SPCh. 6 - Predict the products of the following SN2...Ch. 6 - Prob. 6.46SPCh. 6 - Strawberry growers have used large quantities of...Ch. 6 - A solution of pure (S)-2-iodobutane ([]=+15.90) in...Ch. 6 - Prob. 6.49SPCh. 6 - Give a mechanism to explain the two products...Ch. 6 - Prob. 6.51SPCh. 6 - Because the SN1 reaction goes through a flat...Ch. 6 - Prob. 6.53SPCh. 6 - Furfuryl chloride can undergo substitution by both...Ch. 6 - Prob. 6.55SPCh. 6 - The following reaction takes place under...Ch. 6 - Propose mechanisms to account for the observed...Ch. 6 - Prob. 6.58SPCh. 6 - Prob. 6.59SP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
60. The solar system is 25,000 light years from the center of our Milky Way galaxy. One light year is the dista...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
1. Suppose a chloride ion and a sodium ion are separated by a center—center distance of 5 Å. Is
the interactio...
Biochemistry: Concepts and Connections (2nd Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can you please explain this prooblem to me, show me how the conjugation is added, did I add them in the correct places and if so please show me. Thanks!arrow_forwardBasic strength of organic bases.arrow_forwardNucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See imagearrow_forward
- The answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forwardDraw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forward
- Blocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see imagearrow_forwardElimination-Addition: What molecule was determined to be an intermediate based on a “trapping experiment”? *please solve and see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License