
(a)
Interpretation:
It is to be determined whether the given solvent is suitable for a reaction involving
Concept introduction:
Leveling effects refers to the effect of a solvent on the behavior of acids and bases. If the reactant is a very strong acid or base, it can react with the solvent in an undesired proton transfer reaction. At equilibrium, the strongest acid that can occur in solution is the protonated solvent, and the strongest base that can occur in solution is the deprotonated solvent. For the leveling effect, a solvent is unsuitable for a particular reactant if the reactant (lower
Answer to Problem 6.9P
With respect to the leveling effect, water is not a suitable solvent for a reaction involving
Explanation of Solution
The reaction of
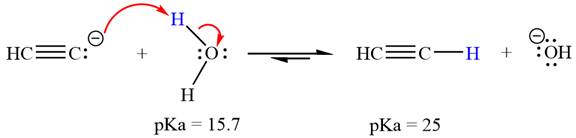
Water,
The solvent effect on the reactant is determined with respect to the leveling effect.
(b)
Interpretation:
It is to be determined whether the given solvent is suitable for a reaction involving
Concept introduction:
Leveling effects refers to the effect of a solvent on the behavior of acids and bases. If the reactant is a very strong acid or base, it can react with the solvent in an undesired proton transfer reaction. At equilibrium, the strongest acid that can occur in solution is the protonated solvent, and the strongest base that can occur in solution is the deprotonated solvent. For the leveling effect, a solvent is unsuitable for a particular reactant if the reactant (lower
Answer to Problem 6.9P
With respect to the leveling effect, ethanol is not a suitable solvent for a reaction involving
Explanation of Solution
The reaction of

Ethanol,
The solvent effect on the reactant is determined with respect to the leveling effect.
(c)
Interpretation:
It is to be determined whether the given solvent is suitable for a reaction involving
Concept introduction:
Leveling effects refers to the effect of a solvent on the behavior of acids and bases. If the reactant is a very strong acid or base, it can react with the solvent in an undesired proton transfer reaction. At equilibrium, the strongest acid that can occur in solution is the protonated solvent, and the strongest base that can occur in solution is the deprotonated solvent. For the leveling effect, a solvent is unsuitable for a particular reactant if the reactant (lower
Answer to Problem 6.9P
With respect to the leveling effect, ethanamide is not a suitable solvent for a reaction involving
Explanation of Solution
The reaction of
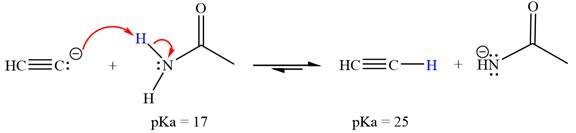
Ethanamide,
The solvent effect on the reactant is determined with respect to the leveling effect.
(d)
Interpretation:
It is to be determined whether the given solvent is suitable for a reaction involving
Concept introduction:
Leveling effects refers to the effect of a solvent on the behavior of acids and bases. If the reactant is a very strong acid or base, it can react with the solvent in an undesired proton transfer reaction. At equilibrium, the strongest acid that can occur in solution is the protonated solvent, and the strongest base that can occur in solution is the deprotonated solvent. For the leveling effect, a solvent is unsuitable for a particular reactant if the reactant (lower
Answer to Problem 6.9P
With respect to the leveling effect,
Explanation of Solution
The reaction of
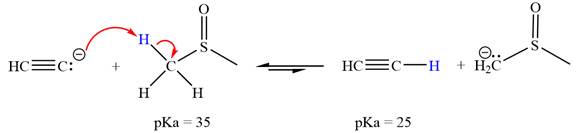
Acetylene,
The solvent effect on the reactant is determined with respect to the leveling effect.
(e)
Interpretation:
It is to be determined whether the given solvent is suitable for a reaction involving
Concept introduction:
Leveling effects refers to the effect of a solvent on the behavior of acids and bases. If the reactant is a very strong acid or base, it can react with the solvent in an undesired proton transfer reaction. At equilibrium, the strongest acid that can occur in solution is the protonated solvent, and the strongest base that can occur in solution is the deprotonated solvent. For the leveling effect, a solvent is unsuitable for a particular reactant if the reactant (lower
Answer to Problem 6.9P
With respect to the leveling effect,
Explanation of Solution
The reaction of
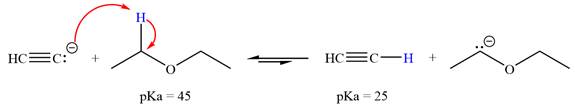
Acetylene,
The solvent effect on the reactant is determined with respect to the leveling effect.
Want to see more full solutions like this?
Chapter 6 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- Show your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers. Something that looks reasonable or correct would be sufficient. If you can get many of them correct that would be great!arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers. Something that looks reasonable or correct would be sufficient. If you can get many of them correct that would be great!arrow_forwardTake a look at the following molecule, and then answer the questions in the table below it. (You can click the other tab to see the molecule without the colored regions.) with colored region plain 0= CH2-0-C-(CH2)16-CH3 =0 CH-O-C (CH2)7-CH=CH-(CH2)5-CH3 D CH3 | + OMPLO CH3-N-CH2-CH2-0-P-O-CH2 B CH3 A Try again * 000 Ar 8 0 ?arrow_forward
- Show your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers.arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers.arrow_forward= 1 = 2 3 4 5 6 ✓ 7 8 ✓ 9 =10 Devise a synthesis to prepare the product from the given starting material. Complete the following reaction scheme. Part 1 of 3 -Br Draw the structure for compound A. Check Step 1 Step 2 A Click and drag to start drawing a structure. × ↓m + OH Save For Later S 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privaarrow_forward
- Predict the products of this organic reduction: 田 Check AP + + H2 Lindlar catalyst Click an drawing 2025 McGraw Hill LLC. All Rigarrow_forward70 Suppose the molecule below is in acidic aqueous solution. Is keto-enol tautomerization possible? • If a keto-enol tautomerization is possible, draw the mechanism for it. Be sure any extra reagents you add to the left-hand sid available in this solution. • If a keto-enol tautomerization is not possible, check the box under the drawing area. : ☐ Add/Remove step Click and drag to st drawing a structure Check Save For Late. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forwardThe problem will not be graded for correctness, but you have to get a reasonable answer something that is either correct or very closer to the correct answer. The instructor professor wants us to do something that shows the answer but everything does not have to be correct. Ideally, yes, it has to be correct. Give it your best shot.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


