
(a)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanamine (
Concept introduction:
Leveling effect refers to the effect of a solvent on the properties of acids and bases. For an acid-base reaction, the strength of the strong acid is limited or leveled by the basicity of the solvent. Similarly, the strength of the strong base is leveled by the acidity of the solvent. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.46P
With respect to the leveling effect, ethanamine is a suitable solvent for a reaction involving the chloride ion
Explanation of Solution
The reaction of chloride ion
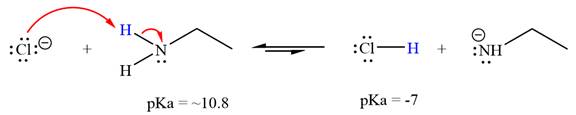
Hydrochloric acid,
The solvent effect on the reactant is determined with respect to the leveling effect.
(b)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanamine (
Concept introduction:
Leveling effect refers to the effect of a solvent on the properties of acids and bases. For an acid-base reaction, the strength of the strong acid is limited or leveled by the basicity of the solvent. Similarly, the strength of the strong base is leveled by the acidity of the solvent. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.46P
With respect to the leveling effect, ethanamine is a not suitable solvent for a reaction involving
Explanation of Solution
The reaction of
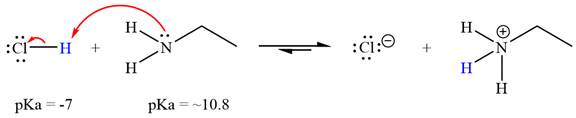
Hydrochloric acid,
The solvent effect on the reactant is determined with respect to the leveling effect.
(c)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanamine (
Concept introduction:
Leveling effect refers to the effect of a solvent on the properties of acids and bases. For an acid-base reaction, the strength of the strong acid is limited or leveled by the basicity of the solvent. Similarly, the strength of the strong base is leveled by the acidity of the solvent. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.46P
With respect to the leveling effect, ethanamine is not a suitable solvent for a reaction involving
Explanation of Solution
The reaction of

Ethanamine,
The solvent effect on the reactant is determined with respect to the leveling effect.
(d)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanamine (
Concept introduction:
Leveling effect refers to the effect of a solvent on the properties of acids and bases. For an acid-base reaction, the strength of the strong acid is limited or leveled by the basicity of the solvent. Similarly, the strength of the strong base is leveled by the acidity of the solvent. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.46P
With respect to the leveling effect, ethanamine is not a suitable solvent for a reaction involving
Explanation of Solution
The reaction of
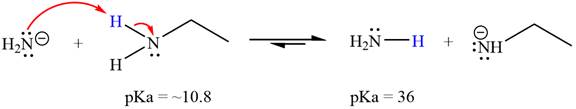
Ethanamine,
The solvent effect on the reactant is determined with respect to the leveling effect.
(e)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanamine (
Concept introduction:
Leveling effect refers to the effect of a solvent on the properties of acids and bases. For an acid-base reaction, the strength of the strong acid is limited or leveled by the basicity of the solvent. Similarly, the strength of the strong base is leveled by the acidity of the solvent. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.46P
With respect to the leveling effect, ethanamine is not a suitable solvent for a reaction involving
Explanation of Solution
The reaction of
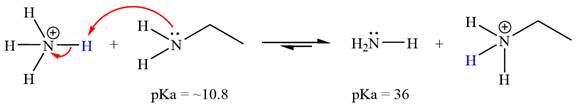
Ethanamine,
The solvent effect on the reactant is determined with respect to the leveling effect.
(f)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanamine ( ) as a solvent with respect to leveling effect.
Concept introduction:
Leveling effect refers to the effect of a solvent on the properties of acids and bases. For an acid-base reaction, the strength of the strong acid is limited or leveled by the basicity of the solvent. Similarly, the strength of the strong base is leveled by the acidity of the solvent. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.46P
With respect to the leveling effect, ethanamine is not a suitable solvent for a reaction involving
Explanation of Solution
The reaction of hydroxide ion
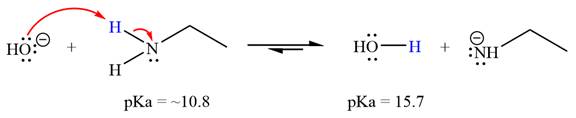
Ethanamine,
The solvent effect on the reactant is determined with respect to the leveling effect.
Want to see more full solutions like this?
Chapter 6 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- For the reaction A (g) → 3 B (g), Kp = 0.379 at 298 K. What is the value of ∆G for this reaction at 298 K when the partial pressures of A and B are 5.70 atm and 0.250 atm?arrow_forward14. Calculate the concentrations of Ag+, Ag(S2O3), and Ag(S2O3)23- in a solution prepared by mixing 150.0 mL of 1.00×10-3 M AgNO3 with 200.0 mL of 5.00 M Na2S2O3 Ag+ + S20 Ag(S203)¯ K₁ = 7.4 × 108 Ag(S203)¯ + S20¯ = Ag(S203) K₂ = 3.9 x 104arrow_forwardΗΝ, cyclohexanone pH 4-5 Draw Enamine I I CH3CH2Br THF, reflux H3O+ I Drawing Draw Iminium Ionarrow_forward
- :0: :0: Select to Add Arrows :0: (CH3)2NH :0: ■ Select to Add Arrows :0: :0: (CH3)2NH ■ Select to Add Arrowsarrow_forwardDraw the product of the following H action sequence. Ignore any inorganic byproducts formed. 1. (CH3CH2)2CuLi, THF 2. CH3Br Q Atoms, Bonds and Rings H Charges ㅁarrow_forwardPlease help me with this the problem is so confusingarrow_forward
- 14 Question (1 point) Disiamylborane adds to a triple bond to give an alkenylborane. Upon oxidation with OH, H2O2, the alkenylborane will form an enol that tautomerizes to an aldehyde. In the first box below, draw the mechanism arrows for the reaction of disiamylborane with the alkyne, and in the last box draw the structure of the aldehyde. 4th attempt Feedback i > 3rd attempt OH, H2O2 i See Periodic Table See Hintarrow_forwardanswer with mechanisms and steps. handwritten please!arrow_forwardHello I need some help with Smartwork. For drawing structure B, I know the correct answer is CH₃B₂, but when I try to type it in, it keeps giving me CH₄BH₃ instead. Do you know how I should write it properly? Should I use a bond or something else?arrow_forward

 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

