
Concept explainers
(a)
Interpretation:
The products of the proton transfer reaction shown below is to be drawn.
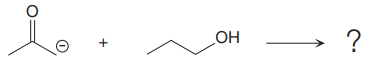
Concept introduction:
In a proton transfer reaction, Bronsted-Lowry base reacts with Bronsted-Lowry acid. Here, Bronsted-Lowry acid donates a proton and forms a conjugate base while Bronsted-Lowry base accepts a proton and forms a conjugate acid. The proton transfer reaction consists of a single elementary step.
(b)
Interpretation:
A free energy diagram for the following reaction, indicating whether it is endothermic or exothermic, is to be drawn.

Concept introduction:
When comparing two acids, the acid with the lower
A reaction tends to be spontaneous if
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- Hi I need help on the question provided in the image.arrow_forwardDraw a reasonable mechanism for the following reaction:arrow_forwardDraw the mechanism for the following reaction: CH3 CH3 Et-OH Et Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron-flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. H± EXP. L CONT. י Α [1] осн CH3 а CH3 :Ö Et H 0 N о S 0 Br Et-ÖH | P LL Farrow_forward
- 20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward20.00 mL of 0.025 M HCl is titrated with 0.035 M KOH. What volume of KOH is needed?arrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
