
Bundle: Chemistry for Engineering Students, 3rd, Loose-Leaf + OWLv2 with QuickPrep 24-Months Printed Access Card
3rd Edition
ISBN: 9781305367388
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 6, Problem 6.8PAE
Interpretation Introduction
Interpretation:
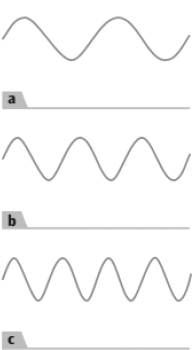
The waves which are depicted here has the highest frequency should be identified. Explain.
Concept introduction:
Frequency is the number of times given wave to pass through a point in a particular time frame, say one second.
Given:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please help me find the 1/Time, Log [I^-] Log [S2O8^2-], Log(time) on the data table. With calculation steps. And the average for runs 1a-1b. Please help me thanks in advance. Will up vote!
Q1: Answer the questions for the reaction below:
..!! Br
OH
a) Predict the product(s) of the reaction.
b) Is the substrate optically active? Are the product(s) optically active as a mix?
c) Draw the curved arrow mechanism for the reaction.
d) What happens to the SN1 reaction rate in each of these instances:
1. Change the substrate to
Br
"CI
2. Change the substrate to
3. Change the solvent from 100% CH3CH2OH to 10% CH3CH2OH + 90% DMF
4. Increase the substrate concentration by 3-fold.
Experiment 27 hates & Mechanisms of Reations
Method I visual Clock Reaction
A. Concentration effects on reaction Rates
Iodine
Run [I] mol/L [S₂082] | Time
mo/L
(SCC)
0.04 54.7
Log
1/ Time Temp Log [ ] 13,20] (time)
/ [I] 199
20.06
23.0
30.04 0.04
0.04 80.0
22.8
45
40.02
0.04 79.0
21.6
50.08
0.03 51.0
22.4
60-080-02 95.0
23.4
7 0.08
0-01 1970
23.4
8 0.08 0.04 16.1
22.6
Chapter 6 Solutions
Bundle: Chemistry for Engineering Students, 3rd, Loose-Leaf + OWLv2 with QuickPrep 24-Months Printed Access Card
Ch. 6 - describe trace analysis and explain its role in...Ch. 6 - describe waves in terms of frequency, wavelength,...Ch. 6 - Prob. 3COCh. 6 - relate the frequency, wavelength, and amplitude of...Ch. 6 - describe the photoelectric effect by stating what...Ch. 6 - Prob. 6COCh. 6 - • use Planck’s equation to calculate the energy of...Ch. 6 - Prob. 8COCh. 6 - Prob. 9COCh. 6 - • describe similarities and differences between...
Ch. 6 - Prob. 11COCh. 6 - Prob. 12COCh. 6 - • identify an orbital (as 1s, 3p, etc.) from its...Ch. 6 - • list the number of orbitals of each type (1s,...Ch. 6 - • sketch the shapes of s and p orbitals and...Ch. 6 - • rank various orbitals in terms of size and...Ch. 6 - Prob. 17COCh. 6 - Prob. 18COCh. 6 - Prob. 19COCh. 6 - Prob. 20COCh. 6 - Prob. 6.1PAECh. 6 - 6.2 Unlike XRF, AAS cannot be used for...Ch. 6 - Prob. 6.3PAECh. 6 - Prob. 6.4PAECh. 6 - Prob. 6.5PAECh. 6 - Prob. 6.6PAECh. 6 - Explain why light is referred to as...Ch. 6 - Prob. 6.8PAECh. 6 - 6.7 Arrange the following regions of the...Ch. 6 - 6.8 Calculate the wavelength in meters, of...Ch. 6 - 6.9 If a string of decorative lights includes...Ch. 6 - 6.10 Define the term refraction.Ch. 6 - 6.11 Define the term photon.Ch. 6 - Prob. 6.14PAECh. 6 - 6.12 Find the energy of a photon with each of the...Ch. 6 - 6.13 Place these types of radiation in order of...Ch. 6 - 6.14 For photon with the following energies,...Ch. 6 - Prob. 6.18PAECh. 6 - 6.16 Various optical disk drives rely on laser...Ch. 6 - 6.17 The laser in most supermarket barcode...Ch. 6 - 6.18 Assume that a microwave oven operates at a...Ch. 6 - 6.19 Fill in the blanks below to complete a...Ch. 6 - 6.20 When light with a wavelength of 58.5 nm...Ch. 6 - 6.21 The electron binding energy fur copper metal...Ch. 6 - What is the difference between continuous and...Ch. 6 - Prob. 6.26PAECh. 6 - 6.23 Describe how the Bohr model of the atom...Ch. 6 - 6.24 According to the Bohr model of the atom, what...Ch. 6 - 6.25 Define the term ground state.Ch. 6 - 6.26 The figure below depicts the first four...Ch. 6 - 6.27 Refer w the data and energy-Ievel diagram...Ch. 6 - 6.28 A neon atom cmi light at many wavelengths,...Ch. 6 - 6.29 A mercury atom emits light at many...Ch. 6 - 6.30 How did the observation of electron...Ch. 6 - 6.31 Why do we use a wave function to describe...Ch. 6 - 6.32 What are the mathematical origins of quantum...Ch. 6 - Prob. 6.37PAECh. 6 - 6.34 Which of the following represent valid sets...Ch. 6 - 6.35 A particular orbital has n = 4 and l = 2....Ch. 6 - 6.36 Why are there no 2d orbitals?Ch. 6 - 6.34 What is the maximum number of electrons in an...Ch. 6 - 6.38 How many orbitals correspond to each of the...Ch. 6 - Prob. 6.43PAECh. 6 - Prob. 6.44PAECh. 6 - 6.40 Referring to Figure 6.15, draw a 4p orbitals,...Ch. 6 - Prob. 6.46PAECh. 6 - 6.43 Define the term spin paired.Ch. 6 - 6.44 On what does the Pauli exclusion principle...Ch. 6 - Prob. 6.49PAECh. 6 - Prob. 6.50PAECh. 6 - Prob. 6.51PAECh. 6 - 6.47 Depict two ways to place electrons in the 2p...Ch. 6 - 6.48 Write the ground state electron configuration...Ch. 6 - 6.49 Which of these electron configurations are...Ch. 6 - 6.50 From the list of atoms and ions given,...Ch. 6 - Prob. 6.56PAECh. 6 - Prob. 6.57PAECh. 6 - Prob. 6.58PAECh. 6 - Describe how valence electron configurations...Ch. 6 - Why is there no element to the immediate right of...Ch. 6 - Prob. 6.61PAECh. 6 - Prob. 6.62PAECh. 6 - 6.55 Explain why the s block of the periodic table...Ch. 6 - Prob. 6.64PAECh. 6 - Prob. 6.65PAECh. 6 - 6.60 Use the electron configurations of the alkali...Ch. 6 - 6.61 Using only a periodic table as a guide,...Ch. 6 - 6.62 Define the term ionization energy....Ch. 6 - 6.63 At which ionization for chlorine would you...Ch. 6 - 6.64 Arrange the following atoms in order of...Ch. 6 - Prob. 6.71PAECh. 6 - 6.66 Which element would you expect to have the...Ch. 6 - Prob. 6.73PAECh. 6 - 6.68 Indicate which species in each pair has the...Ch. 6 - 6.69 Compare the elements Na, B, Al, and C with...Ch. 6 - 6.70 Rank the following in order of decreasing...Ch. 6 - 6.71 Several excited states of the neon atom are...Ch. 6 - Prob. 6.78PAECh. 6 - Prob. 6.79PAECh. 6 - 6.92 The photoelectric effect can he used to...Ch. 6 - 6.93 A mercury atom is initially in its lowest...Ch. 6 - Prob. 6.82PAECh. 6 - 6.95 A metallic sample is known to be barium,...Ch. 6 - 6.96 When a helium atom absorbs light at 58.44 nm,...Ch. 6 - 6.97 Arrange the members of each of the following...Ch. 6 - 6.98 Arrange the following sets of anions in order...Ch. 6 - 6.99 The photoelectric effect can he used in...Ch. 6 - 6.100 Some spacecraft use ion propulsion engines....Ch. 6 - 6.101 Laser welding is a technique in which a...Ch. 6 - Prob. 6.90PAECh. 6 - 6.103 Atomic absorption spectroscopy is based on...Ch. 6 - 6.104 The red color in fireworks is the result of...Ch. 6 - 6.105 When we say that the existence of atomic...Ch. 6 - 6.106 When Bohr devised his model for the atom,...Ch. 6 - Prob. 6.95PAE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (15 pts) Consider the molecule B2H6. Generate a molecular orbital diagram but this time using a different approach that draws on your knowledge and ability to put concepts together. First use VSEPR or some other method to make sure you know the ground state structure of the molecule. Next, generate an MO diagram for BH2. Sketch the highest occupied and lowest unoccupied MOs of the BH2 fragment. These are called frontier orbitals. Now use these frontier orbitals as your basis set for producing LGO's for B2H6. Since the BH2 frontier orbitals become the LGOS, you will have to think about what is in the middle of the molecule and treat its basis as well. Do you arrive at the same qualitative MO diagram as is discussed in the book? Sketch the new highest occupied and lowest unoccupied MOs for the molecule (B2H6).arrow_forwardQ8: Propose an efficient synthesis of cyclopentene from cyclopentane.arrow_forwardQ7: Use compound A-D, design two different ways to synthesize E. Which way is preferred? Please explain. CH3I ONa NaOCH 3 A B C D E OCH3arrow_forward
- Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward(10 pts) The density of metallic copper is 8.92 g cm³. The structure of this metal is cubic close-packed. What is the atomic radius of copper in copper metal?arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward
- Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forwardQ3: Rank the following compounds in increasing reactivity of E1 and E2 eliminations, respectively. Br ca. go do A CI CI B C CI Darrow_forwardQ5: Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2). H₂O דיי "Br KN3 CH3CH2OH NaNH2 NH3 Page 3 of 6 Chem 0310 Organic Chemistry 1 HW Problem Sets CI Br excess NaOCH 3 CH3OH Br KOC(CH3)3 DuckDuckGarrow_forward
- Q4: Circle the substrate that gives a single alkene product in a E2 elimination. CI CI Br Brarrow_forwardPlease calculate the chemical shift of each protonsarrow_forwardQ1: Answer the questions for the reaction below: ..!! Br OH a) Predict the product(s) of the reaction. b) Is the substrate optically active? Are the product(s) optically active as a mix? c) Draw the curved arrow mechanism for the reaction. d) What happens to the SN1 reaction rate in each of these instances: 1. Change the substrate to Br 'CI 2. Change the substrate to 3. Change the solvent from 100% CH3CH2OH to 10% CH3CH2OH + 90% DMF 4. Increase the substrate concentration by 3-fold.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Quantum Numbers, Atomic Orbitals, and Electron Configurations; Author: Professor Dave Explains;https://www.youtube.com/watch?v=Aoi4j8es4gQ;License: Standard YouTube License, CC-BY
QUANTUM MECHANICAL MODEL/Atomic Structure-21E; Author: H to O Chemistry;https://www.youtube.com/watch?v=mYHNUy5hPQE;License: Standard YouTube License, CC-BY