
Concept explainers
Interpretation: The resonance structure of the
Concept Introduction:
- Sometimes the chemical bonding of a molecule cannot be represented using a single Lewis structure. In these cases, the chemical bonding are described by delocalization of electrons and is known as resonance.
- All the possible resonance structures are imaginary whereas the resonance hybrid is real.
- These structures will differ only in the arrangement of the electrons not in the relative position of the atomic nuclei.
To find: The resonance structure of
Answer to Problem 6.62QP
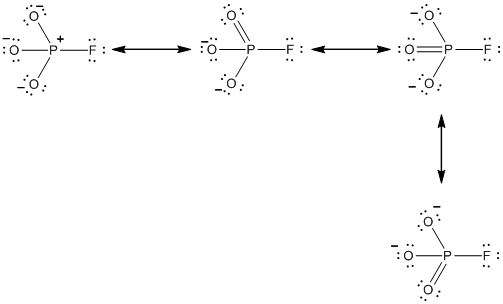
Explanation of Solution
Resonance structure of
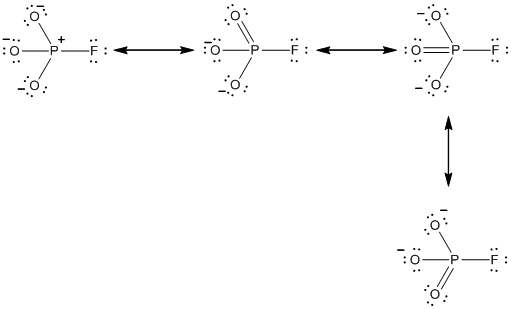
In the case of
The resonance structures of the
Interpretation: The formal charges of the
Concept Introduction
- A formal charge (FC) is the charge assigned to an atom in a molecule, irrespective of relative electronegativity by thinking that electrons in all
chemical bonds are shared equally among atoms. - This method is used to identify the most probable Lewis structures if more than one possibility exists for a compound.
- Formal charge of an atom can be determined by the given formula.
Answer to Problem 6.62QP
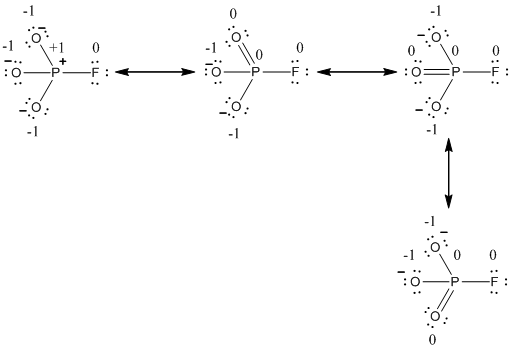
Explanation of Solution
The formal charge of the given resonance structure is given below.
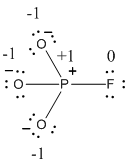
The formal charge of the given resonance structure is calculated,
- Phosphorous atom
Substituting these values to the equation,
- Oxygen atom (a)
Substituting these values to the equation,
- Oxygen atom (b)
Substituting these values to the equation,
- Oxygen atom (c)
Substituting these values to the equation,
- Fluorine atom
Substituting these values to the equation,
The formal charge of the given resonance structure is given below.
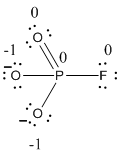
The formal charge of the given resonance structure is calculated,
- Phosphorous atom
Substituting these values to the equation,
- Oxygen atom (a)
Substituting these values to the equation,
- Oxygen atom (b)
Substituting these values to the equation,
- Oxygen atom (c)
Substituting these values to the equation,
- Fluorine atom
Substituting these values to the equation,
The formal charge of the given resonance structure is given below.
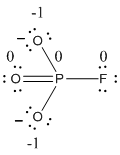
The formal charge of the given resonance structure is calculated,
- Phosphorous atom
Substituting these values to the equation,
- Oxygen atom (a)
Substituting these values to the equation,
- Oxygen atom (b)
Substituting these values to the equation,
- Oxygen atom (c)
Substituting these values to the equation,
- Fluorine atom
Substituting these values to the equation,
The formal charge of the given resonance structure is given below.
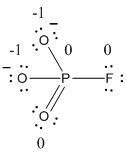
The formal charge of the given resonance structure is calculated,
- Phosphorous atom
Substituting these values to the equation,
- Oxygen atom (a)
Substituting these values to the equation,
- Oxygen atom (b)
Substituting these values to the equation,
- Oxygen atom (c)
Substituting these values to the equation,
- Fluorine atom
Substituting these values to the equation,
The formal charge of the
Want to see more full solutions like this?
Chapter 6 Solutions
CHEMISTRY: ATOMS FIRST VOL 1 W/CONNECT
- + C8H16O2 (Fatty acid) + 11 02 → 8 CO2 a. Which of the above are the reactants? b. Which of the above are the products? H2o CO₂ c. Which reactant is the electron donor? Futty acid d. Which reactant is the electron acceptor? e. Which of the product is now reduced? f. Which of the products is now oxidized? 02 #20 102 8 H₂O g. Where was the carbon initially in this chemical reaction and where is it now that it is finished? 2 h. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forward→ Acetyl-CoA + 3NAD+ + 1FAD + 1ADP 2CO2 + CoA + 3NADH + 1FADH2 + 1ATP a. Which of the above are the reactants? b. Which of the above are the products? c. Which reactant is the electron donor? d. Which reactants are the electron acceptors? e. Which of the products are now reduced? f. Which product is now oxidized? g. Which process was used to produce the ATP? h. Where was the energy initially in this chemical reaction and where is it now that it is finished? i. Where was the carbon initially in this chemical reaction and where is it now that it is finished? j. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. OCH 3 (Choose one) OH (Choose one) Br (Choose one) Explanation Check NO2 (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Aarrow_forward
- For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects O donating O withdrawing O no inductive effects Resonance Effects Overall Electron-Density ○ donating ○ withdrawing O no resonance effects O electron-rich O electron-deficient O similar to benzene Cl O donating O withdrawing ○ donating ○ withdrawing O no inductive effects O no resonance effects O Explanation Check O electron-rich O electron-deficient similar to benzene X © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forwardIdentifying electron-donating and For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects NH2 ○ donating NO2 Explanation Check withdrawing no inductive effects Resonance Effects Overall Electron-Density ○ donating O withdrawing O no resonance effects O donating O withdrawing O donating withdrawing O no inductive effects Ono resonance effects O electron-rich electron-deficient O similar to benzene O electron-rich O electron-deficient O similar to benzene olo 18 Ar 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation Check Х (Choose one) OH (Choose one) OCH3 (Choose one) OH (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward
- Assign R or S to all the chiral centers in each compound drawn below porat bg 9 Br Brarrow_forwarddescrive the energy levels of an atom and howan electron moces between themarrow_forwardRank each set of substituents using the Cahn-Ingold-Perlog sequence rules (priority) by numbering the highest priority substituent 1.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


