
Concept explainers
Draw Lewis structures for the following four isoelectronic species: (a) CO, (b) NO+, (c) CN−, (d) N2. Show formal charges. (See Problem 6.69.)
(a)
Interpretation: The Lewis structures for the given isoelectronic species should be shown.
Concept Introduction:
- Lewis structures are diagrams that represent the chemical bonding of covalently bonded molecules and coordination compounds.
- It is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
- The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell.
To find: The Lewis structure for the given set of isoelectronic species.
Answer to Problem 6.83QP

Explanation of Solution
Given isoelectronic species is below.
Lewis structure of the above isoelectronic species is drawn below.

The total number of valence electrons is found to be 10, where carbon and oxygen has 4 and 6 valence electrons respectively.
The 8 electrons getting after reducing two electrons for each bond from the total valence electron are distributed on nitrogen atom to complete the octet.
Since the octets of carbon atoms are not filled, a triple bond was made between carbon and oxygen atoms in expense of two electrons where the remaining four electrons are distributed equally over two atoms present in the given species.
(b)
Interpretation: The Lewis structures and the formal charges for the given isoelectronic species should be shown.
Concept Introduction:
- Lewis structures are diagrams that represent the chemical bonding of covalently bonded molecules and coordination compounds.
- It is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
- The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell.
To find: The Lewis structure for the given set of isoelectronic species.
Answer to Problem 6.83QP
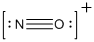
Explanation of Solution
Given isoelectronic species is below.
Lewis structure of the above isoelectronic species is drawn below.

The total number of valence electrons is found to be 11, where nitrogen and oxygen contains 5 and 6 valence electrons respectively. The whole charge of the molecule is +1 that results in the total number of valence electrons as 10.
The 8 electrons getting after reducing two electrons for each bond from the total valence electron are distributed on nitrogen atom to complete the octet.
Since the octets of nitrogen atoms are not filled, a triple bond is made between nitrogen and oxygen atoms in expense of two electrons where the remaining four electrons are distributed over the 2 atoms present in the given molecule.
(c)
Interpretation: The Lewis structures and the formal charges for the given isoelectronic species should be shown.
Concept Introduction:
- Lewis structures are diagrams that represent the chemical bonding of covalently bonded molecules and coordination compounds.
- It is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
- The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell.
To find: The Lewis structure for the given set of isoelectronic species.
Answer to Problem 6.83QP

Explanation of Solution
Given isoelectronic molecule is below.
Lewis structure of the above isoelectronic species is drawn below.

The total number of valence electrons is found to be 9, where nitrogen and carbon contributes 5 and 4 electrons respectively. The whole charge of the molecule is -1 making the total number of valence electrons 10.
The 8 electrons getting after reducing two electrons for each bond from the total valence electron are distributed on nitrogen atom to complete the octet.
Since the octets of carbon atoms are not filled, a triple bond is made between carbon and nitrogen atoms in expense of two electrons where the remaining four electrons are distributed over the atoms present in the given molecule.
(d)
Interpretation: The Lewis structures and the formal charges for the given isoelectronic species should be shown.
Concept Introduction:
- Lewis structures are diagrams that represent the chemical bonding of covalently bonded molecules and coordination compounds.
- It is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
- The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell.
To find: The Lewis structure for the given set of isoelectronic species.
Answer to Problem 6.83QP

Explanation of Solution
Given isoelectronic species is below.
Lewis structure of above isoelectronic species is drawn below.

The total number of valence electrons is found to be 10, where both nitrogen atoms contribute 5 electrons.
The 8 electrons getting after reducing two electrons for each bond from the total valence electron are distributed on nitrogen atom to complete the octet.
Since the octets of nitrogen atoms are not filled, a triple bond is made between both nitrogen atoms.
(a)
Interpretation: The formal charges for the given isoelectronic species should be shown.
Concept Introduction
A formal charge (FC) is the charge assigned to an atom in a molecule, irrespective of relative electronegativity by thinking that electrons in all chemical bonds are shared equally among atoms.
This method is used to identify the most probable Lewis structures if more than one possibility exists for a compound.
The Lewis structure with formal charge on each of the atoms close to zero is taken as the most plausible structure.
Formal charge of an atom can be determined by the given formula.
Answer to Problem 6.83QP
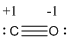
Explanation of Solution
Formal charge of the given isoelectronic species is given below
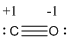
The formal charge of the given isoelectronic species is calculated,
- Carbon atom
Substituting these values to the equation,
- Oxygen atom
Substituting these values to the equation,
(b)
Interpretation: The formal charges for the given isoelectronic species should be shown.
Concept Introduction
A formal charge (FC) is the charge assigned to an atom in a molecule, irrespective of relative electronegativity by thinking that electrons in all chemical bonds are shared equally among atoms.
This method is used to identify the most probable Lewis structures if more than one possibility exists for a compound.
The Lewis structure with formal charge on each of the atoms close to zero is taken as the most plausible structure.
Formal charge of an atom can be determined by the given formula.
Answer to Problem 6.83QP
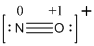
Explanation of Solution
Formal charge of the given isoelectronic species is given below
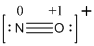
The formal charge of the given isoelectronic species is calculated,
- Nitrogen atom
Substituting these values to the equation,
- Oxygen atom
Substituting these values to the equation,
(c)
Interpretation: The formal charges for the given isoelectronic species should be shown.
Concept Introduction
A formal charge (FC) is the charge assigned to an atom in a molecule, irrespective of relative electronegativity by thinking that electrons in all chemical bonds are shared equally among atoms.
This method is used to identify the most probable Lewis structures if more than one possibility exists for a compound.
The Lewis structure with formal charge on each of the atoms close to zero is taken as the most plausible structure.
Formal charge of an atom can be determined by the given formula.
Answer to Problem 6.83QP
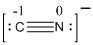
Explanation of Solution
Formal charge of the given isoelectronic species is given below
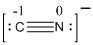
The formal charge of the given isoelectronic species is calculated,
- Carbon atom
Substituting these values to the equation,
- Nitrogen atom
Substituting these values to the equation,
(d)
Interpretation: The formal charges for the given isoelectronic species should be shown.
Concept Introduction
A formal charge (FC) is the charge assigned to an atom in a molecule, irrespective of relative electronegativity by thinking that electrons in all chemical bonds are shared equally among atoms.
This method is used to identify the most probable Lewis structures if more than one possibility exists for a compound.
The Lewis structure with formal charge on each of the atoms close to zero is taken as the most plausible structure.
Formal charge of an atom can be determined by the given formula.
Answer to Problem 6.83QP
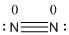
Explanation of Solution
Formal charge of the given isoelectronic species is given below
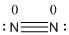
The formal charge of the given isoelectronic species is calculated,
- Nitrogen atom
Substituting these values to the equation,
- Since both the nitrogen atoms are similar, the formal charge of the nitrogen atoms is zero.
Want to see more full solutions like this?
Chapter 6 Solutions
CHEMISTRY: ATOMS FIRST VOL 1 W/CONNECT
- b. CH3 H3C CH3 CH3 H3C an unexpected product, containing a single 9- membered ring the expected product, containing two fused rings H3C-I (H3C)2CuLi an enolatearrow_forwardb. H3C CH3 1. 2. H3O+ H3C MgBr H3Carrow_forwardPredict the major products of this reaction: excess H+ NaOH ? A Note that the first reactant is used in excess, that is, there is much more of the first reactant than the second. If there won't be any products, just check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privarrow_forward
- 1. For each of the reaction "railroads" below, you are either asked to give the structure(s) of the starting material(s) or product(s), or provide reagents/conditions to accomplish the transformation, as indicated by the boxes. a. NaOMe H+ .CO,H HO₂C MeOH (excess) MeOH H3C Br يع CH3 1. LiAlH4 2. H3O+ 3. PBг3 H3C 1. Et-Li 2. H3O+ -CO₂Me -CO₂Me OH CH3 CH3 ল CH3arrow_forwardPredict the intermediate 1 and final product 2 of this organic reaction: NaOMe ག1, ད།་, - + H You can draw 1 and 2 in any arrangement you like. 2 work up Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds at the chiral center. Explanation Check Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Parrow_forwardWhat is the total energy cost associated with the compound below adopting the shown conformation? CH3 HH DH CH3arrow_forward
- ΗΝ, Draw Final Product C cyclohexanone pH 4-5 Edit Enamine H3O+ CH3CH2Br THF, reflux H Edit Iminium Ionarrow_forwardHow many hydrogen atoms are connected to the indicated carbon atom?arrow_forwardIdentify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forward
- Identify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





