
Concept explainers
(a)
Interpretation:
The products of the proton transfer reaction shown below are to be drawn.
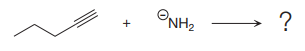
Concept introduction:
In proton transfer reaction, a Bronsted-Lowry base reacts with a Bronsted-Lowry acid. The Bronsted-Lowry acid donates a proton and forms a conjugate base while the Bronsted-Lowry base accepts a proton and forms a conjugate acid. The proton transfer reaction consists of a single elementary step.
(b)
Interpretation:
A free energy diagram for the following reaction, indicating whether it is endothermic or exothermic, is to be drawn.
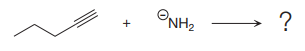
Concept introduction:
When comparing two acids, the acid with the lower
A reaction tends to be spontaneous if
Trending nowThis is a popular solution!

Chapter 6 Solutions
Get Ready for Organic Chemistry
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
