
(a)
Interpretation:
The products for the given proton transfer reaction are to be drawn and the favored equilibrium side with numerical factor is to be determined.
Concept introduction:
The proton transfer reactions favor the side opposite the stronger acid. Larger the
Answer to Problem 6.41P
The products for the given reaction are:

The equilibrium is favored to product side by a factor of
Explanation of Solution
The given reaction is:
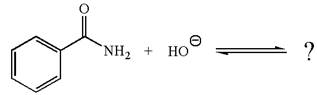
The ion

In the conjugate base formed, the negative charge on nitrogen is delocalized through the electron withdrawing resonance effect of carbonyl group. Thus, amide is a stronger acid than water, and hence, the equilibrium is favored to the product side.
The
The favored equilibrium side with numerical value is determined on the basis of stronger acid and
(b)
Interpretation:
The products for the given proton transfer reaction are to be drawn and the favored equilibrium side with the numerical factor is to be determined.
Concept introduction:
The proton transfer reactions favor the side opposite the stronger acid. Larger the
Answer to Problem 6.41P
The products for the given reaction are:
The equilibrium is favored to the reactant side by a factor of
Explanation of Solution
The given reaction is:
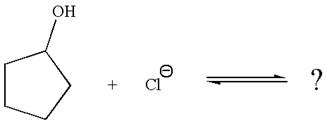
In the given reaction,
The favored equilibrium side with numerical value is determined on the basis of the stronger acid and
(c)
Interpretation:
The products for the given proton transfer reaction are to be drawn and the favored equilibrium side with numerical factor is to be determined.
Concept introduction:
The proton transfer reactions favor the side opposite the stronger acid. Larger the
Answer to Problem 6.41P
The products for the given reaction are:
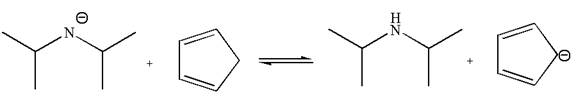
The equilibrium is favored to product side by a factor of
Explanation of Solution
The given reaction is:
In the given reaction, cyclopentadiene acts as an acid and the negatively charge nitrogen abstracts a proton from diisopropylamine to give the following products:
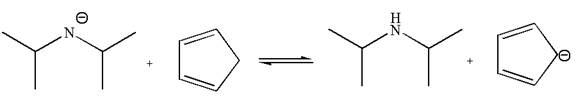
On the product side, the negative charge on carbon is a resonance stabilized by a conjugated double bond; such stabilization of the negative charge is not possible on the reactant side where the negative charge is on nitrogen bonded to two electron donating isopropyl groups. The acid is stronger when its conjugate base is stable, therefore, cyclopentadiene is a stronger acid than
According to Appendix
The favored equilibrium side with numerical value is determined on the basis of stronger acid and
(d)
Interpretation:
The products for the given proton transfer reaction are to be drawn and the favored equilibrium side with numerical factor is to be determined.
Concept introduction:
The proton transfer reactions favor the side opposite the stronger acid. Larger the
Answer to Problem 6.41P
The products for the given reaction are:

The equilibrium is favored to the product side by a factor of
Explanation of Solution
The given reaction is:
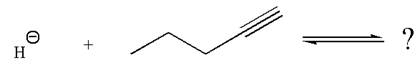
In the given reaction, the hydride ion abstracts the terminal proton of an

As the effective electronegativity of
According to Appendix
The favored equilibrium side with numerical value is determined on the basis of stronger acid and
(e)
Interpretation:
The products for the given proton transfer reaction are to be drawn and the favored equilibrium side with numerical factor is to be determined.
Concept introduction:
The proton transfer reactions favor the side opposite the stronger acid. Larger the
Answer to Problem 6.41P
The products for the given reaction are:

The equilibrium is favored to the product side by a factor of
Explanation of Solution
The given reaction is:
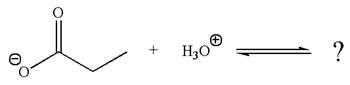
In the given reaction, the propanoate ion abstracts the proton of hydronium ion to give the following products:

According to Appendix
The favored equilibrium side with numerical value is determined on the basis of stronger acid and
(f)
Interpretation:
The products for the given proton transfer reaction are to be drawn and the favored equilibrium side with numerical factor is to be determined.
Concept introduction:
The proton transfer reactions favor the side opposite the stronger acid. Larger the
Answer to Problem 6.41P
The products for the given reaction are:

The equilibrium is favored to the product side. Benzene is the weaker acid by a factor of
Explanation of Solution
The given reaction is:
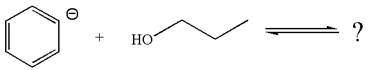
In the given reaction, the

As the oxygen atom is more electronegative than carbon, the negative charge on oxygen is more stable as compared to carbon. Thus, an anion on the right side, having negative charge on oxygen, is more stable than the anion on the left side where the negative charge is on carbon. Therefore, propanol is more acidic than benzene, and hence, the reaction is favored to the product side.
According to Appendix
The favored equilibrium side with numerical value is determined on the basis of stronger acid and the
(g)
Interpretation:
The products for the given proton transfer reaction are to be drawn and the favored equilibrium side with numerical factor is to be determined.
Concept introduction:
The proton transfer reactions favor the side opposite the stronger acid. Larger the
Answer to Problem 6.41P
The products for the given reaction are:

The equilibrium is favored to the product side. Benzene is the weaker acid by a factor of
Explanation of Solution
The given reaction is:
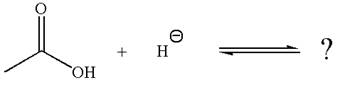
In the given reaction, the hydride ion abstracts the proton from carboxylic acid and gives the following products:

The conjugate base formed with a negative charge on the oxygen atom is better stabilized by the resonance effect. This makes the carboxylic acid the stronger acid, and the equilibrium is favored to the product side.
According to Appendix
The favored equilibrium side with numerical value is determined on the basis of stronger acid and the
Want to see more full solutions like this?
Chapter 6 Solutions
Get Ready for Organic Chemistry
- Nonearrow_forwardIn the phase diagram of steel (two components Fe and C), region A is the gamma austenite solid and region B contains the gamma solid and liquid. Indicate the degrees of freedom that the fields A and B have,arrow_forwardFor a condensed binary system in equilibrium at constant pressure, indicate the maximum number of phases that can exist.arrow_forward
- Part V. Label ad match the carbons in compounds Jane and Diane w/ the corresponding peak no. in the Spectra (Note: use the given peak no. To label the carbons, other peak no are intentionally omitted) 7 4 2 -0.13 -0.12 -0.11 -0.10 -0.08 8 CI Jane 1 -0.09 5 210 200 190 180 170 160 150 140 130 120 110 100 -8 90 f1 (ppm) 11 8 172.4 172.0 f1 (ppr HO CI NH Diane 7 3 11 80 80 -80 -R 70 60 60 2 5 -8 50 40 8. 170 160 150 140 130 120 110 100 90 -0 80 70 20 f1 (ppm) 15 30 -20 20 -60 60 -0.07 -0.06 -0.05 -0.04 -0.03 -0.02 -0.01 -0.00 -0.01 10 -0.17 16 15 56 16 -0.16 -0.15 -0.14 -0.13 -0.12 -0.11 -0.10 -0.09 -0.08 -0.07 -0.06 -0.05 -0.04 17.8 17.6 17.4 17.2 17.0 f1 (ppm) -0.03 -0.02 550 106 40 30 20 20 -0.01 -0.00 F-0.01 10 0arrow_forwardConsider the reaction of 2-methylpropane with a halogen. With which halogen will the product be almost exclusively 2-halo-2-methylpropane? 1. F2 2. Cl2 3. Br2 4. I2arrow_forwardNonearrow_forward
- Nonearrow_forwardn Feb 3 A T + 4. (2 pts) Draw the structure of the major component of the Limonene isolated. Explain how you confirmed the structure. 5. (2 pts) Draw the fragment corresponding to the base peak in the Mass spectrum of Limonene. 6. (1 pts) Predict the 1H NMR spectral data of R-Limonene. Proton NMR: 5.3 pon multiplet (H Ringarrow_forwardPart VI. Ca H 10 O is the molecular formula of compound Tom and gives the in the table below. Give a possible structure for compound Tom. 13C Signals summarized C1 C2 C3 C4 C5 C6 C7 13C shift (ppm) 23.5 27.0 33.0 35.8 127 162 205 DEPT-90 + DEPT-135 + +arrow_forward
- 2. Using the following data to calculate the value of AvapH o of water at 298K. AvapH o of water at 373K is 40.7 kJ/mol; molar heat capacity of liquid water at constant pressure is 75.2J mol-1 K-1 and molar heat capacity of water vapor at constant pressure is 33.6 J mol-1 K-1.arrow_forwardPart VII. Below are the 'HNMR 13 3 C-NMR, COSY 2D- NMR, and HSQC 20-NMR (Similar with HETCOR but axes are reversed) spectra of an organic compound with molecular formula C6H13 O. Assign chemical shift values to the H and c atoms of the compound. Find the structure. Show complete solutions. Predicted 1H NMR Spectrum ли 4.7 4.6 4.5 4.4 4.3 4.2 4.1 4.0 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 2.8 2.7 2.6 2.5 2.4 2.3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8 f1 (ppm)arrow_forward3. Draw the expanded structural formula, the condensed structural formula, and the skeletal structural formula for 2-pentene. expanded structure: Condensed structure: Skeletal formula: 4. Draw the expanded structural formula, the condensed structural formula, and the skeletal structural formula for 2-methyl-3-heptene. expanded structure: Condensed structure: Skeletal formula: following structurearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

