
A fuel cell is an electrochemical device in which hydrogen reacts with oxygen to produce water and DC electricity. A 1-watt proton-exchange membrane fuel cell (PEMFC) could be used for portable applications such as cellular telephones, and a 100-kW PEMFC could be used to power an automobile
The following reactions occur inside the PEMFC:
Anode:
Cathode:
Overall:
A ?owchart of a single cell of a PEMFC is shown below. The complete cell would consist of a stack of such cells in series, such as the one shown in Problem 9.19.
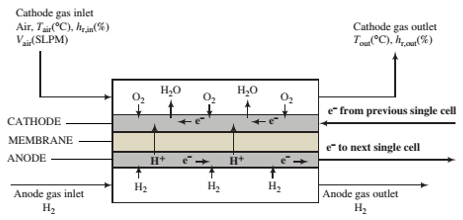
The cell consists of two gas channels separated by a membrane sandwiched between two ?at carbon-paper electrodes—the anode and the cathode—that contain imbedded platinum particles. Hydrogen ?ows into the anode chamber and contacts the anode, where H2molecules are catalyzed by the platinum to dissociate and ionize to form hydrogen ions (protons) and electrons. The electrons are conducted through the carbon ?bers of the anode to an external circuit, where they pass to the cathode of the next cell in the stack. The hydrogen ions permeate from the anode through the membrane to the cathode.
Humid air is fed into the cathode chamber, and at the cathode O2molecules are catalytically split to form oxygen atoms, which combine with the hydrogen ions coming through the membrane and electrons coming from the external circuit to form water. The water desorbs into the cathode gas and is carried out of the cell. The membrane material is a hydrophilic polymer that absorbs water molecules and facilitates the transport of the hydrogen ions from the anode to the cathode. Electrons come from the anode of the cell at one end of the stack and ?ow through an external circuit to drive the device that the fuel cell is powering, while the electrons coming from the device ?ow back to the cathode at the opposite end of the stack to complete the circuit.
It is important to keep the water content of the cathode gas between upper and lower limits. lf the content reaches a value for which the relative humidity would exceed 100%, condensation occurs at the cathode (?ooding), and the entering oxygen must diffuse through a liquid water ?lm before it cart react. The rate of this diffusion is much lower than the rate of diffusion through the gas film normally adjacent to the cathode, and so the performance of the fuel cell deteriorates. On the other hand, if there is not enough water in the cathode gas (less than 85% relative humidity), the membrane dries out and cannot transport hydrogen efficiently, which also leads to reduced performance.
A 400-cell 300—volt PEMFC operates at steady state with a power output of 36 kW. The air fed to the cathode side is at 200°C and roughly 1.0 atm (absolute) with a relative humidity of 70.0% and a volumetric ?ow rate of
(a) Explain in your own words what happens in a single cell of a PEMFC.
(b) The stoichiometric hydrogen requirement for a PEMFC is given by
(c) Use the expression of Part (b) to determine the molar rates of oxygen consumed and water generated in the unit with the given speci?cations, both in units of mol/min. (Remember that power = voltage × current.) Then determine the relative humidity of the cathode exit stream,
(d) Determine the minimum cathode inlet ?ow rate in SLPM to prevent the fuel cell from ?ooding
Learn your wayIncludes step-by-step video

Chapter 6 Solutions
ELEMENTARY PRINCIPLES OF CHEM. PROCESS.
Additional Science Textbook Solutions
Modern Database Management
Starting Out With Visual Basic (8th Edition)
Starting Out with C++ from Control Structures to Objects (9th Edition)
Starting Out with Java: From Control Structures through Objects (7th Edition) (What's New in Computer Science)
Starting Out with Java: From Control Structures through Data Structures (4th Edition) (What's New in Computer Science)
Introduction To Programming Using Visual Basic (11th Edition)
- #3 Orthonitroanaline (an important intermediate in dyes - called fast orange) is formed from the reaction of orthonitroanaline (ONCB) and aqueous ammonia. This liquid phase reaction is first order in both ONCB and ammonia with k = 0.0017 m³/kmol·min at 188 °C. The initial entering concentration of ONCB and ammonia are 1.8 kmol/m³ and 6.6 kmol/m³, respectively. ONCB is used as the basis of calculation. NO2 CI NO2 NH₂ + NHCI +2NH₂ a) Express the concentration of each species solely as a function of conversion.arrow_forward4. (15 pts)A chemical project with a fixed capital investment without land of $250,000. The operation of the chemical project starts at the end of year 1 with 8-years of project lifetime. The estimated revenue per year is $90,000, the estimated cost of manufacture without depreciation over the project lifetime is $30,000/yr, and the taxation rate is 40%. a. Please determine the yearly depreciation values using the standard MACRS method assuming surplus value of $5,000. b. Please determine the net profit for operation year 1, 5, and 8.arrow_forward2. (10 pts) You got a loan of $300,000 from a bank for your new house at a yearly interest rate of 6%, compounded monthly. How much do you pay total to the bank if the loan is 15 years? How much do you pay total to the bank if the loan is 30 years? 3. (10 pts) You got a 5-year loan of $50,000 to buy a BMW car at a yearly interest rate of 6% Please calculate your monthly payment if it is compounded monthly? Please calculate your quarterly payment if it is compounded quarterly?arrow_forward
- A buffer solution is made by mixing 0.1 M acetic acid (HA) and 0.05 M sodium acetate (A⁻). The pKa of acetic acid is 4.76. Due to an experimental error, the actual pH was not recorded, and we need to solve for the concentration of the conjugate base (A⁻) given that the desired pH should be 4.90. Use the Bisection Method to find the concentration of A.arrow_forward1. (15) John had an loan plan shown in the following discrete cash flow diagram: $4,000 $6,000 GI $2,000 5 7 1 2 3 4 $3,000 $4,000 ? Years a. Please describe this diagram in terms of borrowing and payback. b. How much does John need to pay to totally payoff the loan at the end of year 8 if the interest rate is 8%? c. If John pays the sam amount of money at year 8, how much can John borrow at year 0 without paying back in between with the same interest rate?arrow_forwardA buffer solution is made by mixing 0.1 M acetic acid (HA) and 0.05 M sodium acetate (A⁻). The pKa of acetic acid is 4.76. Due to an experimental error, the actual pH was not recorded, and we need to solve for the concentration of the conjugate base (A⁻) given that the desired pH should be 4.90. Use the Bisection Method to find the concentration of A.arrow_forward
- 1. Liquid heptane is stored in a 100,000-L storage vessel that is vented directly to air. The heptane is stored at 25°C and 1 atm pressure. The liquid is drained from the storage vessel and all that remains in the vessel is the air saturated with heptane vapor. a. Is the vapor in the storage vessel flammable? b. What is the TNT equivalent for the vapor remaining in the vessel? c. If the vapor explodes, what is the overpressure 50 m from the vessel? d. What damage can be expected at 50 m?arrow_forward2. You have decided to use a vacuum purging technique to purge oxygen from a reactor vessel to reduce the concentration to 2.0% (mol). The reactor is 18 ft diameter and 40 ft tall. The temperature is 80°F. Assume that the vacuum purge goes from atmospheric pressure to 10.0 psia. How many purge cycles are required and how many total moles of nitrogen must be used? Assume the purge is done with pure nitrogen. 3. If the purging described in problem 2 takes place using nitrogen that has 1% (mol) oxygen in it, how many vacuum purge cycles are required? How many total moles of the inert gas must be used? 4. If the purging described in problem 2 is done by way of a "sweep-through" purge instead of a vacuum purge, for how long (in minutes) must the inert gas flow through the vessel if there is a 20 psig supply of pure nitrogen available at 150 CFM (ft³/min)? How much nitrogen must be used (lbm)?arrow_forward5. Look at Figure 7-14. Determine the voltage developed between the steel nozzle and the grounded vessel, and how much energy is stored in the nozzle. Explain the potential hazards for cases A and B from the following table: Case A Case B Hose length (ft) 75 75 Hose diameter (in) 2.0 2.0 Flow rate (gpm) 30 30 Liquid conductivity (mho/cm) 2x10-8 1x10-14 Dielectric constant 2.3 25 Density (g/cm³) 0.8 0.9 6. In Problem 5, case B, what would be the most effective way to reduce the potential hazards in this situation?arrow_forward
- 2. You have decided to use a vacuum purging technique to purge oxygen from a reactor vessel to reduce the concentration to 2.0% (mol). The reactor is 18 ft diameter and 40 ft tall. The temperature is 80°F. Assume that the vacuum purge goes from atmospheric pressure to 10.0 psia. How many purge cycles are required and how many total moles of nitrogen must be used? Assume the purge is done with pure nitrogen.arrow_forwardAn 8-foot ion exchange bed needs to be backwashed with water to remove impurities. The particles have a density of 1.24 g/cm³ and an average size of 1.1 mm. Calculate: a. The minimum fluidization velocity using water at 30°C? b. The velocity required to expand the bed by 30%? Assumptions: The ion exchange bed particles are spherical (sphericity = 1.1), and the minimum fluidization porosity (ɛM) is 0.3. Notes: At 30°C, the viscosity (μ) of water is 0.797 cP, and the density (ρ) is 0.995 g/cm³.arrow_forwardfluidized bed reactor uses a solid catalyst with a particle diameter of 0.25 mm, a bulk density of 1.50 g/mL, and a sphericity of 0.90. Under packed bed conditions, the porosity is 0.35, and the bed height is 2 m. The gas enters from the bottom of the reactor at a temperature of 600°C, with a viscosity of 0.025 cP and a density of 0.22 lb/ft³. At minimum fluidization, the porosity reaches 0.45. Calculate: a. The minimum superficial velocity (VM) of the gas entering the fluidized column. b. The bed height if V = 2 VM c. The pressure drop under conditions where V =2.5 VMarrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





