
Concept explainers
MATHEMATICAL For the following aspartase reaction (see Question 28) in the presence of the inhibitor hydroxymethylaspartate, determine
Interpretation:
The
Concept introduction:
In an enzymatic reaction, the inhibitors are the molecules that bind with the enzymes and block their activity. Enzymatic inhibition can be of many types. If a substrate and an inhibitor compete with each other to bind with the enzyme and both have similar structures, then the inhibition is known as competitive. On the other hand, if an inhibitor does not compete with the substrate and both of them can bind to an enzyme at their respective specific sites, then it is known as non-competitive inhibition.
Answer to Problem 61RE
Plot the reciprocal substrate concentration on the x-axis and two reaction velocities (both in the presence and absence of inhibitors) on the y-axis.
Before drawing the plot, the data needs to be modified so that it can be aligned in the Lineweaver–Burk plot. The modification of the data is as follows:
The Lineweaver–Burk plot from above data is:
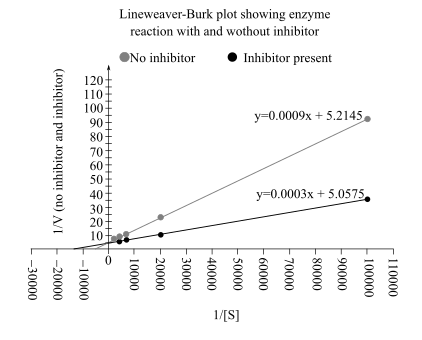
From this plot
In an uninhibited reaction, the
Therefore, the value of
Therefore, the value of
By comparing the Lineweaver–Burk plot of both reactions, it can be said that the inhibition is competitive in nature
Explanation of Solution
Given information:
In competitive inhibition, the substrate and the inhibitor have a similar structure. Hence, like the substrate, the inhibitor has also got nearly same affinity toward the active site of an enzyme and is able to bind with the enzyme. As a result, the enzyme’s affinity towards the substrate changes (it inhibits the substrate to bind with the enzyme). In other words, the
Also, in Lineweaver–Burk plot the
The reaction (without inhibitor) has a low value of
Want to see more full solutions like this?
Chapter 6 Solutions
Biochemistry
- The following data were recorded for the enzyme catalyzed conversion of S -> P. Question: Estimate the Vmax and Km. What would be the rate at 2.5 and 5.0 x 10-5 M [S] ?arrow_forwardPlease helparrow_forwardThe following data were recorded for the enzyme catalyzed conversion of S -> P Question: what would the rate be at 5.0 x 10-5 M [S] and the enzyme concentration was doubled? Also, the rate given in the table is from product accumulation after 10 minuets of reaction time. Verify these rates represent a true initial rate (less than 5% turnover). Please helparrow_forward
- The following data was obtained on isocitrate lyase from an algal species. Identify the reaction catalyzed by this enzyme, deduce the KM and Vmax , and determine the nature of the inhibition by oxaloacetate. Please helparrow_forwardIn the table below, there are sketches of four crystals made of positively-charged cations and negatively-charged anions. Rank these crystals in decreasing order of stability (or equivalently increasing order of energy). That is, select "1" below the most stable (lowest energy) crystal. Select "2" below the next most stable (next lowest energy) crystal, and so forth. A B 鹽 (Choose one) +2 C +2 +2 (Choose one) D 鹽雞 (Choose one) (Choose one)arrow_forward1. Draw the structures for the fats A. 16:2: w-3 and B. 18:3:49,12,15 2. Name each of the molecules below (image attached)arrow_forward
- draw the structures for the fats A. 16:2:w-3 B 18:3:9,12,15arrow_forward1. Below is a template strand of DNA. Show the mRNA and protein that would result. label the ends of the molecules ( refer to attached image)arrow_forwardAttach the followina labels to the diagram below: helicase, single stranded binding proteins, lagging strand, leading strand, DNA polymerase, primase, 5' ends (3), 3' ends (3) (image attached)arrow_forward
- 1. How much energy in terms of ATP can be obtained from tristearin (stearate is 18:0) Show steps pleasearrow_forwardMultiple choice urgent!!arrow_forward1. Write the transamination reaction for alanine. Indicate what happens next to each of the molecules in the reaction, and under what conditions it happens. 2.arrow_forward
 BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
