
(a)
Interpretation:Outcome of the below transformation should be explained mechanistically.

Concept introduction: Bimolecular substitution or
A general
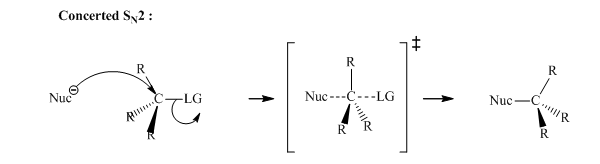
Polar-aprotic solvents accelerate the rate of
(b)
Interpretation:Outcome of the below transformation should be explained mechanistically.
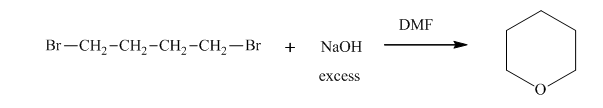
Concept introduction: Bimolecular substitution or
A general
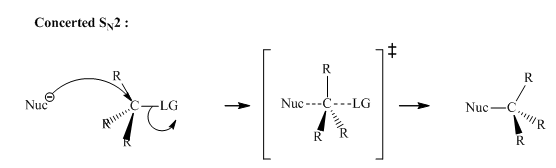
Polar-aprotic solvents accelerate the rate of
(c)
Interpretation:Outcome of the below transformation should be explained mechanistically.
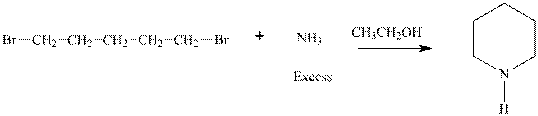
Concept introduction: Bimolecular substitution or
A general
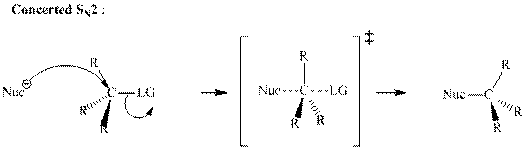
Polar-aprotic solvents accelerate the rate of
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic Chemistry: Structure and Function
- What is the hydronium ion concentration in a solution of water that has a hydroxide ion concentrationof 1.0 x 10-2 M?arrow_forwardIdentify conjugate acid-base pairs in the following reactions:HBr (aq) + H2O (l) ⇌ H3O+ (aq) + Br- (aq) - OH (aq) + CH3COOH (aq) ⇌ H2O (l) + CH3COO- (aq)arrow_forward4:45 PM Tue Apr 1 K 77% Problem 9 of 10 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting structure, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Then draw any missing organic intermediates or products for this reaction. Include all lone pairs in the structures. Ignore inorganic byproducts, counterions, and solvents. :0: H Select to Add Arrows HI CH3OH H+ ·HO CH3OH, H+ 0:0 H H Select to Add Arrows tion Versirate CH3OH, H* Select to Draw Productarrow_forward
- Can I please get help with this graph? If you can show exactly where it needs to pass through.arrow_forwardG 1. PPh3, THF 2. 3. LiH, THF ' THF H Harrow_forwardPlease EnCircle or Fill-In your Choice CLEARLY: 21. Please Sketch the intermediates for each step below. Draw the Product which would result from the following series of reactions. Name each Type of Rx: 1. Br2, FeBr3 2. Mg, ether 3. ethylene oxide 4. H₂O+ 5. PBr3 6. Mg, ether 7. 8. H3O+, heat (-H₂O 9. HF ?arrow_forward
- Can I please get help with this question. All required information should be in data table.arrow_forwardesc For the reaction below: 1. Draw all reasonable elimination products to the right of the arrow. 2. In the box below the reaction, redraw any product you expect to be a major product. Major Product: Explanation Check C ☐ + X NaOH Br F1 F2 80 F3 F4 F5 F6 1 ! @ 2 3 $ 4 % 5 Q W LU E S D A F7 * C Click and dr drawing a 2025 McGraw Hill LLC. All Rights Reserv ►II F8 4 F9 6 7 8 9 R T Y U LL F G H Jarrow_forwardCalculate equilibrium concentrations for the following reaction:N2 (g) + O2 (g) ⇋ 2 NO (g) Kc = 0.10 at 2273K initially [N2] = 0.200M; [O2] = 0.200arrow_forward
- For each scenario below, select the color of the solution using the indicator thymol blue during the titration. When you first add indicator to your Na2CO3solution, the solution is basic (pH ~10), and the color is ["", "", "", "", ""] . At the equivalence point for the titration, the moles of added HCl are equal to the moles of Na2CO3. One drop (or less!) past this is called the endpoint. The added HCl begins to titrate the thymol blue indicator itself. At the endpoint, the indicator color is ["", "", "", "", ""] . When you weren't paying attention and added too much HCl (~12 mL extra), the color is ["", "", "", "", ""] . When you really weren't paying attention and reached the second equivalence point of Na2CO3, the color isarrow_forwardThe following reaction is run in which the initial conditions include only methane (CH4) at a concentration of0.115 M. Once equilibrium was established, the concentration of acetylene (C2H2) was measured to be 0.035M. What is the value of the equilibrium constant, K?2 CH4 (g) ⇋ C2H2 (g) + 3 H2 (g)arrow_forwardCalculate the equilibrium concentration of carbon dioxide for the following reaction:2 COF2 (g) ⇋ CF4 (g) + CO2 (g) Kc = 2.00 at 10.00 °C. at equilibrium [COF2] = 0.255M; [CF4] = 0.118Marrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

