
General Chemistry
4th Edition
ISBN: 9781891389603
Author: Donald A. McQuarrie, Peter A. Rock, Ethan B. Gallogly
Publisher: University Science Books
expand_more
expand_more
format_list_bulleted
Question
Chapter 6, Problem 6.19P
(a)
Interpretation Introduction
Interpretation:
The older name of
Concept Introduction:
In the older nomenclature system, an “–ous” ending is used to indicate the lower common ionic charge, and an “–ic” ending is used to indicate the higher common ionic charge.
Some of the examples are shown below,
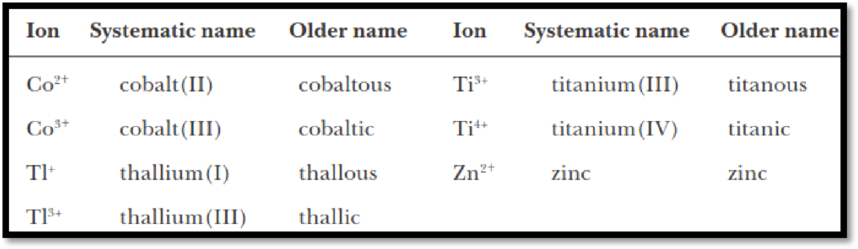
Figure 1
(b)
Interpretation Introduction
Interpretation:
The older name of
Concept Introduction:
Refer to part (a).
(c)
Interpretation Introduction
Interpretation:
The older name of
Concept Introduction:
Refer to part (a).
(d)
Interpretation Introduction
Interpretation:
The older name of
Concept Introduction:
Refer to part (a).
(e)
Interpretation Introduction
Interpretation:
The older name of
Concept Introduction:
Refer to part (a).
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Choose the Lewis structure for the compound below:
H2CCHOCH2CH(CH3)2
HH
H
:d
H
H
H C.
Η
H
H
HH
H
H
H
H.
H
H
H
HH
H
H
H
H
H-
H
H
H
C-H
H
H
HHHH
Each of the highlighted carbon atoms
is connected to
hydrogen atoms.
く
Complete the reaction in the drawing area below by adding the major products to the right-hand side.
If there won't be any products, because nothing will happen under these reaction conditions, check the box under the drawing area
instead.
Note: if the products contain one or more pairs of enantiomers, don't worry about drawing each enantiomer with dash and wedge
bonds. Just draw one molecule to represent each pair of enantiomers, using line bonds at the chiral center.
More...
No reaction.
Explanation
Check
O
+
G
1. Na O Me
Click and drag to start
drawing a structure.
2. H
+
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
000
Ar
P
Chapter 6 Solutions
General Chemistry
Ch. 6 - Prob. 6.1PCh. 6 - Prob. 6.2PCh. 6 - Prob. 6.3PCh. 6 - Prob. 6.4PCh. 6 - Prob. 6.5PCh. 6 - Prob. 6.6PCh. 6 - Prob. 6.7PCh. 6 - Prob. 6.8PCh. 6 - Prob. 6.9PCh. 6 - Prob. 6.10P
Ch. 6 - Prob. 6.11PCh. 6 - Prob. 6.12PCh. 6 - Prob. 6.13PCh. 6 - Prob. 6.14PCh. 6 - Prob. 6.15PCh. 6 - Prob. 6.16PCh. 6 - Prob. 6.17PCh. 6 - Prob. 6.18PCh. 6 - Prob. 6.19PCh. 6 - Prob. 6.20PCh. 6 - Prob. 6.21PCh. 6 - Prob. 6.22PCh. 6 - Prob. 6.23PCh. 6 - Prob. 6.24PCh. 6 - Prob. 6.25PCh. 6 - Prob. 6.26PCh. 6 - Prob. 6.27PCh. 6 - Prob. 6.28PCh. 6 - Prob. 6.29PCh. 6 - Prob. 6.30PCh. 6 - Prob. 6.31PCh. 6 - Prob. 6.32PCh. 6 - Prob. 6.33PCh. 6 - Prob. 6.34PCh. 6 - Prob. 6.35PCh. 6 - Prob. 6.36PCh. 6 - Prob. 6.37PCh. 6 - Prob. 6.38PCh. 6 - Prob. 6.39PCh. 6 - Prob. 6.40PCh. 6 - Prob. 6.41PCh. 6 - Prob. 6.42PCh. 6 - Prob. 6.43PCh. 6 - Prob. 6.44PCh. 6 - Prob. 6.45PCh. 6 - Prob. 6.46PCh. 6 - Prob. 6.47PCh. 6 - Prob. 6.48PCh. 6 - Prob. 6.49PCh. 6 - Prob. 6.50PCh. 6 - Prob. 6.51PCh. 6 - Prob. 6.52PCh. 6 - Prob. 6.53PCh. 6 - Prob. 6.54PCh. 6 - Prob. 6.55PCh. 6 - Prob. 6.56PCh. 6 - Prob. 6.57PCh. 6 - Prob. 6.58PCh. 6 - Prob. 6.59PCh. 6 - Prob. 6.60PCh. 6 - Prob. 6.61PCh. 6 - Prob. 6.62PCh. 6 - Prob. 6.63PCh. 6 - Prob. 6.64PCh. 6 - Prob. 6.65PCh. 6 - Prob. 6.66PCh. 6 - Prob. 6.67PCh. 6 - Prob. 6.68PCh. 6 - Prob. 6.69PCh. 6 - Prob. 6.70PCh. 6 - Prob. 6.71PCh. 6 - Prob. 6.72PCh. 6 - Prob. 6.73PCh. 6 - Prob. 6.74PCh. 6 - Prob. 6.75PCh. 6 - Prob. 6.76PCh. 6 - Prob. 6.77PCh. 6 - Prob. 6.78P
Knowledge Booster
Similar questions
- Draw a tetramer of this alternating copolymer.arrow_forwardH I T H HH H -H C. H- Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H CIH H H H HHHH H H 0 ·H H– 冊 CH CHI HH C- H- H H- H H A. H H C H H- -H HH H B. H- -H D. H H H H • H -H E. -H H H HICH T HHH F. H-arrow_forwardPolylactic acid (shown below) is a biodegradable polymer used for food packaging. Identify the monomer(s) used in the production of this polymer using a condensation process.arrow_forward
- Draw the product of the reaction shown below. Ignore small byproducts that would evaporate pleasearrow_forwardPoly(ethylene adipate) is a biodegradable polyester (shown below). Identify the type of polymerization process used in the production of this polymer.arrow_forwardPolymers may be composed of thousands of monomers. draw two repeat units(dimer) of the polymer formed in this reaction. assume there are hydrogen atoms on the two ends of the dimer. ignore inorganic byproducts pleasearrow_forward
- Draw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproductsarrow_forwardDraw the product of this reaction please. Ignore inorganic byproductsarrow_forwardOne of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forward
- Draw the monomers required to synthesize this condensation polymer please.arrow_forwardProvide the correct systematic name for the compound shown here. Please take into account the keyboard options belowarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY