
Chemistry
13th Edition
ISBN: 9781259911156
Author: Raymond Chang Dr., Jason Overby Professor
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 6, Problem 6.144QP
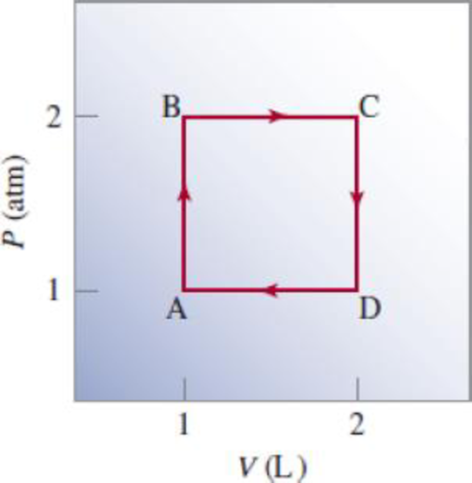
Starting at A, an ideal gas undergoes a cyclic process involving expansion and compression, as shown here. Calculate the total work done. Does your result support the notion that work is not a state function?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Identifying the major species in weak acid or weak base equilibria
The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at
equilibrium. You can leave out water itself.
Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the
formulas of the species that will act as neither acids nor bases in the 'other' row.
You will find it useful to keep in mind that HF is a weak acid.
acids:
0.2 mol of KOH is added to
1.0 L of a 0.5 M HF
solution.
bases:
Х
other: ☐
acids:
0.10 mol of HI is added to
1.0 L of a solution that is
1.4M in both HF and NaF.
bases:
other: ☐
0,0,...
ด
?
18
Ar
Identifying the major species in weak acid or weak base equilibria
The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at
equilibrium. You can leave out water itself.
Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the
formulas of the species that will act as neither acids nor bases in the 'other' row.
You will find it useful to keep in mind that NH3 is a weak base.
acids: ☐
1.8 mol of HCl is added to
1.0 L of a 1.0M NH3
bases: ☐
solution.
other: ☐
0.18 mol of HNO3 is added
to 1.0 L of a solution that is
1.4M in both NH3 and
NH₁Br.
acids:
bases: ☐
other: ☐
0,0,...
?
000
18
Ar
B
1
Using reaction free energy to predict equilibrium composition
Consider the following equilibrium:
2NH3 (g) = N2 (g) +3H₂
—N2 (g) AGº = 34. kJ
Now suppose a reaction vessel is filled with 4.19 atm of ammonia (NH3) and 9.94 atm of nitrogen (N2) at 378. °C. Answer the following questions about this
system:
rise
Under these conditions, will the pressure of NH 3 tend to rise or fall?
☐ x10
fall
Х
Is it possible to reverse this tendency by adding H₂?
In other words, if you said the pressure of NH 3 will tend to rise, can that
be changed to a tendency to fall by adding H₂? Similarly, if you said the
pressure of NH3 will tend to fall, can that be changed to a tendency to
rise by adding H₂?
If you said the tendency can be reversed in the second question, calculate
the minimum pressure of H₂ needed to reverse it.
Round your answer to 2 significant digits.
yes
no
atm
00.
18
Ar
무ㅎ
?
Chapter 6 Solutions
Chemistry
Ch. 6.2 - Classify each of the following as an open system,...Ch. 6.2 - Determine if the following processes are...Ch. 6.3 - A gas expands from 264 mL to 971 mL at constant...Ch. 6.3 - A gas expands and does P-V work on the...Ch. 6.3 - Two ideal gases at the same temperature and...Ch. 6.3 - Calculate the work done when a gas at a pressure...Ch. 6.3 - Prob. 3RCFCh. 6.4 - Calculate the heat evolved when 266 g of white...Ch. 6.4 - What is U for the formation of 1 mole of CO at 1...Ch. 6.4 - Which of the constant-pressure processes shown...
Ch. 6.4 - Given the thermochemical equation...Ch. 6.4 - Calculate U for the following reaction at 1 atm...Ch. 6.5 - An iron bar of mass 869 g cools from 94C to 5C....Ch. 6.5 - A quantity of 1.922 g of methanol (CH3OH) was...Ch. 6.5 - A 30.14-g stainless steel ball bearing at 117.82C...Ch. 6.5 - A quantity of 4.00 102 mL of 0.600 M HNO3 is...Ch. 6.5 - A 1-g sample of Al and a 1-g sample of Fe are...Ch. 6.5 - A 1.252 g-sample of cyclohexanol (C6H12O) was...Ch. 6.5 - A 100.0-g sample of an unknown metal at 125C is...Ch. 6.6 - Calculate the standard enthalpy of formation of...Ch. 6.6 - Benzene (C6H6) burns in air to produce carbon...Ch. 6.6 - Which of the following does not have Hfo=0 at 25C?...Ch. 6.6 - Explain why reactions involving reactant compounds...Ch. 6.6 - Using data from Appendix 2, calculate Hrxno for...Ch. 6.6 - Given the following information...Ch. 6.7 - Use the data in Appendix 2 to calculate the heat...Ch. 6 - Define these terms: system, surroundings, open...Ch. 6 - What is heat? How does heat differ from thermal...Ch. 6 - What are the units for energy commonly employed in...Ch. 6 - A truck initially traveling at 60 km per hour is...Ch. 6 - These are various forms of energy: chemical, heat,...Ch. 6 - Define these terms: thermochemistry, exothermic...Ch. 6 - Stoichiometry is based on the law of conservation...Ch. 6 - Describe two exothermic processes and two...Ch. 6 - Decomposition reactions are usually endothermic,...Ch. 6 - On what law is the first law of thermodynamics...Ch. 6 - Explain what is meant by a state function. Give...Ch. 6 - The internal energy of an ideal gas depends only...Ch. 6 - Consider these changes: (a) Hg(l)Hg(g) (b)...Ch. 6 - A sample of nitrogen gas expands in volume from...Ch. 6 - A gas expands in volume from 26.7 mL to 89.3 mL at...Ch. 6 - A gas expands and does P-V work on the...Ch. 6 - The work done to compress a gas is 74 J. As a...Ch. 6 - Calculate the work done when 50.0 g of tin...Ch. 6 - Calculate the work done in joules when 1.0 mole of...Ch. 6 - Prob. 6.21QPCh. 6 - In writing thermochemical equations, why is it...Ch. 6 - Explain the meaning of this thermochemical...Ch. 6 - Consider this reaction:...Ch. 6 - The first step in the industrial recovery of zinc...Ch. 6 - Determine the amount of heat (in kJ) given off...Ch. 6 - Consider the reaction...Ch. 6 - Consider the reaction...Ch. 6 - What is the difference between specific heat and...Ch. 6 - Define calorimetry and describe two commonly used...Ch. 6 - Consider the following data: Metal Al Cu Mass (g)...Ch. 6 - A piece of silver of mass 362 g has a heat...Ch. 6 - A 6.22-kg piece of copper metal is heated from...Ch. 6 - Calculate the amount of heat liberated (in kJ)...Ch. 6 - A sheet of gold weighing 10.0 g and at a...Ch. 6 - To a sample of water at 23.4C in a...Ch. 6 - A 0.1375-g sample of solid magnesium is burned in...Ch. 6 - A quantity of 85.0 mL of 0.900 M HCl is mixed with...Ch. 6 - What is meant by the standard-state condition?Ch. 6 - How are the standard enthalpies of an element and...Ch. 6 - What is meant by the standard enthalpy of a...Ch. 6 - Write the equation for calculating the enthalpy of...Ch. 6 - State Hesss law. Explain, with one example, the...Ch. 6 - Describe how chemists use Hesss law to determine...Ch. 6 - Which of the following standard enthalpy of...Ch. 6 - The Hfo values of the two allotropes of oxygen, O2...Ch. 6 - Which is the more negative quantity at 25C: Hfo...Ch. 6 - Predict the value of Hfo (greater than, less than,...Ch. 6 - In general, compounds with negative Hfo values are...Ch. 6 - Suggest ways (with appropriate equations) that...Ch. 6 - Calculate the heat of decomposition for this...Ch. 6 - The standard enthalpies of formation of ions in...Ch. 6 - Calculate the heats of combustion for the...Ch. 6 - Calculate the heats of combustion for the...Ch. 6 - Methanol, ethanol, and n-propanol are three common...Ch. 6 - The standard enthalpy change for the following...Ch. 6 - From the standard enthalpies of formation,...Ch. 6 - Pentaborane-9, B5H9, is a colorless, highly...Ch. 6 - Determine the amount of heat (in kJ) given off...Ch. 6 - At 850C, CaCO3 undergoes substantial decomposition...Ch. 6 - From these data,...Ch. 6 - From the following data,...Ch. 6 - From the following heats of combustion,...Ch. 6 - Calculate the standard enthalpy change for the...Ch. 6 - Prob. 6.65QPCh. 6 - Why is the lattice energy of a solid always a...Ch. 6 - Consider two ionic compounds A and B. A has a...Ch. 6 - Mg2+ is a smaller cation than Na+ and also carries...Ch. 6 - Why is it dangerous to add water to a concentrated...Ch. 6 - Which of the following does not have Hfo=O at 25C?...Ch. 6 - Calculate the expansion work done when 3.70 moles...Ch. 6 - Prob. 6.73QPCh. 6 - Given the thermochemical equations:...Ch. 6 - The standard enthalpy change H for the thermal...Ch. 6 - Hydrazine, N2H4, decomposes according to the...Ch. 6 - A quantity of 2.00 102 mL of 0.862 M HCl is mixed...Ch. 6 - A 3.53-g sample of ammonium nitrate (NH4NO3) was...Ch. 6 - Consider the reaction...Ch. 6 - Prob. 6.80QPCh. 6 - Prob. 6.81QPCh. 6 - A 2.10-mole sample of crystalline acetic acid,...Ch. 6 - Prob. 6.83QPCh. 6 - You are given the following data:...Ch. 6 - A gaseous mixture consists of 28.4 mole percent of...Ch. 6 - When 2.740 g of Ba reacts with O2 at 298 K and 1...Ch. 6 - Methanol (CH3OH) is an organic solvent and is also...Ch. 6 - A 44.0-g sample of an unknown metal at 99.0C was...Ch. 6 - Using the data in Appendix 2, calculate the...Ch. 6 - Producer gas (carbon monoxide) is prepared by...Ch. 6 - Prob. 6.91QPCh. 6 - Prob. 6.92QPCh. 6 - Ethanol (C2H5OH) and gasoline (assumed to be all...Ch. 6 - The combustion of what volume of ethane (C2H6),...Ch. 6 - Prob. 6.95QPCh. 6 - Prob. 6.96QPCh. 6 - Explain the cooling effect experienced when...Ch. 6 - For which of the following reactions does...Ch. 6 - Prob. 6.99QPCh. 6 - A quantity of 0.020 mole of a gas initially at...Ch. 6 - Prob. 6.101QPCh. 6 - Prob. 6.102QPCh. 6 - Prob. 6.103QPCh. 6 - Prob. 6.104QPCh. 6 - A person ate 0.50 pound of cheese (an energy...Ch. 6 - Prob. 6.106QPCh. 6 - Prob. 6.107QPCh. 6 - The enthalpy of combustion of benzoic acid...Ch. 6 - Prob. 6.109QPCh. 6 - Prob. 6.110QPCh. 6 - Glaubers salt, sodium sulfate decahydrate (Na2SO4 ...Ch. 6 - A balloon 16 m in diameter is inflated with helium...Ch. 6 - Acetylene (C2H2) can be hydrogenated (reacting...Ch. 6 - Prob. 6.114QPCh. 6 - An excess of zinc metal is added to 50.0 mL of a...Ch. 6 - (a) A person drinks four glasses of cold water...Ch. 6 - Prob. 6.118QPCh. 6 - Why are cold, damp air and hot, humid air more...Ch. 6 - Prob. 6.120QPCh. 6 - Prob. 6.121QPCh. 6 - Prob. 6.122QPCh. 6 - Prob. 6.123QPCh. 6 - Determine the standard enthalpy of formation of...Ch. 6 - Prob. 6.125QPCh. 6 - Ice at 0C is placed in a Styrofoam cup containing...Ch. 6 - Prob. 6.127QPCh. 6 - Prob. 6.128QPCh. 6 - Calculate the internal energy of a Goodyear blimp...Ch. 6 - Prob. 6.131QPCh. 6 - Acetylene (C2H2) can be made by reacting calcium...Ch. 6 - The average temperature in deserts is high during...Ch. 6 - From a thermochemical point of view, explain why a...Ch. 6 - Calculate the U for the following reaction at 298...Ch. 6 - Lime is a term that includes calcium oxide (CaO,...Ch. 6 - A 4.117-g impure sample of glucose (C6H12O6) was...Ch. 6 - Construct a table with the headings q, w, U, and...Ch. 6 - The combustion of 0.4196 g of a hydrocarbon...Ch. 6 - Metabolic activity in the human body releases...Ch. 6 - Give an example for each of the following...Ch. 6 - From the following data, calculate the heat of...Ch. 6 - Starting at A, an ideal gas undergoes a cyclic...Ch. 6 - For reactions in condensed phases (liquids and...Ch. 6 - The diagrams (a)(d) represent various physical and...Ch. 6 - A 20.3-g sample of an unknown metal and a 28.5-g...Ch. 6 - Prob. 6.148QPCh. 6 - Prob. 6.149QPCh. 6 - The fastest serve in tennis is about 150 mph. Can...Ch. 6 - Prob. 6.151QPCh. 6 - It has been estimated that 3 trillion standard...Ch. 6 - Prob. 6.153QPCh. 6 - Prob. 6.154QPCh. 6 - Prob. 6.155QPCh. 6 - We hear a lot about how the burning of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Some people consider Pasteur or Koch to be the Father of Microbiology, rather than Leeuwenhoek. Why might they ...
Microbiology with Diseases by Body System (5th Edition)
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
More than one choice may apply. Using the terms listed below, fill in the blank with the proper term. anterior ...
Essentials of Human Anatomy & Physiology (12th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. 2.2 mol of NaOH is added to 1.0 L of a 1.4M HF solution. acids: П bases: Х other: ☐ ப acids: 0.51 mol of KOH is added to 1.0 L of a solution that is bases: 1.3M in both HF and NaF. other: ☐ 00. 18 Ararrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: N2O4 (g) 2NO2 (g) AG⁰ = 5.4 kJ Now suppose a reaction vessel is filled with 1.68 atm of dinitrogen tetroxide (N204) at 148. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of N2O4 tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO2? In other words, if you said the pressure of N2O4 will tend to rise, can that be changed to a tendency to fall by adding NO2? Similarly, if you said the pressure of N2O4 will tend to fall, can that be changed to a tendency to rise by adding NO2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO 2 needed to reverse it. Round your answer to 2 significant digits. yes no 0.42 atm ☑ 5 0/5 ? مله Ararrow_forwardHomework 13 (Ch17) Question 4 of 4 (1 point) | Question Attempt: 2 of 2 ✓ 1 ✓ 2 = 3 4 Time Remaining: 4:25:54 Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free energy of the following chemical reaction: 2CH3OH (g)+302 (g) → 2CO2 (g) + 4H₂O (g) Round your answer to zero decimal places. ☐ kJ x10 ☐ Subm Check 2020 Hill LLC. All Rights Reserved. Terms of Use | Privacy Cearrow_forward
- Identifying the major species in weak acid or weak base equilibria Your answer is incorrect. • Row 2: Your answer is incorrect. • Row 3: Your answer is incorrect. • Row 6: Your answer is incorrect. 0/5 The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. acids: HF 0.1 mol of NaOH is added to 1.0 L of a 0.7M HF solution. bases: 0.13 mol of HCl is added to 1.0 L of a solution that is 1.0M in both HF and KF. Exponent other: F acids: HF bases: F other: K 1 0,0,... ? 000 18 Ararrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NOCI (g) 2NO (g) + Cl2 (g) AGº =41. kJ Now suppose a reaction vessel is filled with 4.50 atm of nitrosyl chloride (NOCI) and 6.38 atm of chlorine (C12) at 212. °C. Answer the following questions about this system: ? rise Under these conditions, will the pressure of NOCI tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO? In other words, if you said the pressure of NOCI will tend to rise, can that be changed to a tendency to fall by adding NO? Similarly, if you said the pressure of NOCI will tend to fall, can that be changed to a tendency to rise by adding NO? yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO needed to reverse it. Round your answer to 2 significant digits. 0.035 atm ✓ G 00. 18 Ararrow_forwardHighlight each glycosidic bond in the molecule below. Then answer the questions in the table under the drawing area. HO- HO- -0 OH OH HO NG HO- HO- OH OH OH OH NG OHarrow_forward
- € + Suppose the molecule in the drawing area below were reacted with H₂ over a platinum catalyst. Edit the molecule to show what would happen to it. That is, turn it into the product of the reaction. Also, write the name of the product molecule under the drawing area. Name: ☐ H C=0 X H- OH HO- H HO- -H CH₂OH ×arrow_forwardDraw the Haworth projection of the disaccharide made by joining D-glucose and D-mannose with a ẞ(1-4) glycosidic bond. If the disaccharide has more than one anomer, you can draw any of them. Click and drag to start drawing a structure. Xarrow_forwardEpoxides can be opened in aqueous acid or aqueous base to produce diols (molecules with two OH groups). In this question, you'll explore the mechanism of epoxide opening in aqueous acid. 2nd attempt Be sure to show all four bonds at stereocenters using hash and wedge lines. 0 0 Draw curved arrows to show how the epoxide reacts with hydronium ion. 100 +1: 1st attempt Feedback Be sure to show all four bonds at stereocenters using hash and wedge lines. See Periodic Table See Hint H A 5 F F Hr See Periodic Table See Hintarrow_forward
- 03 Question (1 point) For the reaction below, draw both of the major organic products. Be sure to consider stereochemistry. > 1. CH₂CH₂MgBr 2. H₂O 3rd attempt Draw all four bonds at chiral centers. Draw all stereoisomers formed. Draw the structures here. e 130 AN H See Periodic Table See Hint P C Brarrow_forwardYou may wish to address the following issues in your response if they are pertinent to the reaction(s) you propose to employ:1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Please make it in detail and draw it out too in what step what happens. Thank you for helping me!arrow_forward1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Everything in detail and draw out and write it.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY