
Chemistry
13th Edition
ISBN: 9781259911156
Author: Raymond Chang Dr., Jason Overby Professor
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 6.146QP
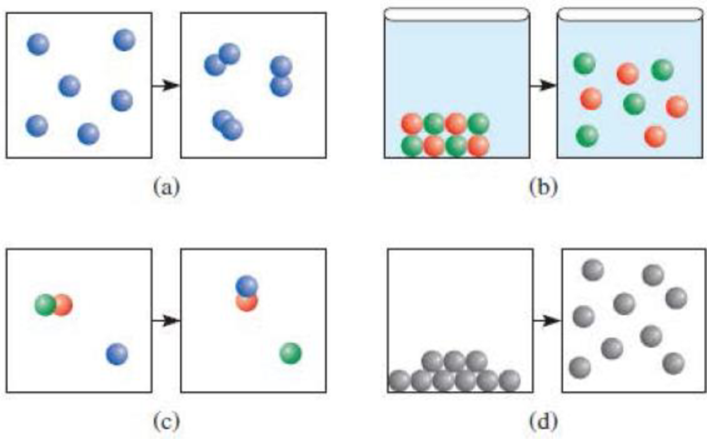
The diagrams (a)–(d) represent various physical and chemical processes: (a) 2A(g) → A2(g); (b) MX(s) → M+(aq) + X−(aq); (c) AB(g) + C(g) → AC(g) + B(g); (d) B(l) → B(g). Predict whether the situations shown are endothermic or exothermic. Explain why in some cases no clear conclusions can be made.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the total energy cost associated with the compound below adopting the shown conformation?
CH3
HH
DH
CH3
ΗΝ,
Draw Final Product
C
cyclohexanone
pH 4-5
Edit Enamine
H3O+
CH3CH2Br
THF, reflux
H
Edit Iminium Ion
How many hydrogen atoms are connected to the indicated carbon atom?
Chapter 6 Solutions
Chemistry
Ch. 6.2 - Classify each of the following as an open system,...Ch. 6.2 - Determine if the following processes are...Ch. 6.3 - A gas expands from 264 mL to 971 mL at constant...Ch. 6.3 - A gas expands and does P-V work on the...Ch. 6.3 - Two ideal gases at the same temperature and...Ch. 6.3 - Calculate the work done when a gas at a pressure...Ch. 6.3 - Prob. 3RCFCh. 6.4 - Calculate the heat evolved when 266 g of white...Ch. 6.4 - What is U for the formation of 1 mole of CO at 1...Ch. 6.4 - Which of the constant-pressure processes shown...
Ch. 6.4 - Given the thermochemical equation...Ch. 6.4 - Calculate U for the following reaction at 1 atm...Ch. 6.5 - An iron bar of mass 869 g cools from 94C to 5C....Ch. 6.5 - A quantity of 1.922 g of methanol (CH3OH) was...Ch. 6.5 - A 30.14-g stainless steel ball bearing at 117.82C...Ch. 6.5 - A quantity of 4.00 102 mL of 0.600 M HNO3 is...Ch. 6.5 - A 1-g sample of Al and a 1-g sample of Fe are...Ch. 6.5 - A 1.252 g-sample of cyclohexanol (C6H12O) was...Ch. 6.5 - A 100.0-g sample of an unknown metal at 125C is...Ch. 6.6 - Calculate the standard enthalpy of formation of...Ch. 6.6 - Benzene (C6H6) burns in air to produce carbon...Ch. 6.6 - Which of the following does not have Hfo=0 at 25C?...Ch. 6.6 - Explain why reactions involving reactant compounds...Ch. 6.6 - Using data from Appendix 2, calculate Hrxno for...Ch. 6.6 - Given the following information...Ch. 6.7 - Use the data in Appendix 2 to calculate the heat...Ch. 6 - Define these terms: system, surroundings, open...Ch. 6 - What is heat? How does heat differ from thermal...Ch. 6 - What are the units for energy commonly employed in...Ch. 6 - A truck initially traveling at 60 km per hour is...Ch. 6 - These are various forms of energy: chemical, heat,...Ch. 6 - Define these terms: thermochemistry, exothermic...Ch. 6 - Stoichiometry is based on the law of conservation...Ch. 6 - Describe two exothermic processes and two...Ch. 6 - Decomposition reactions are usually endothermic,...Ch. 6 - On what law is the first law of thermodynamics...Ch. 6 - Explain what is meant by a state function. Give...Ch. 6 - The internal energy of an ideal gas depends only...Ch. 6 - Consider these changes: (a) Hg(l)Hg(g) (b)...Ch. 6 - A sample of nitrogen gas expands in volume from...Ch. 6 - A gas expands in volume from 26.7 mL to 89.3 mL at...Ch. 6 - A gas expands and does P-V work on the...Ch. 6 - The work done to compress a gas is 74 J. As a...Ch. 6 - Calculate the work done when 50.0 g of tin...Ch. 6 - Calculate the work done in joules when 1.0 mole of...Ch. 6 - Prob. 6.21QPCh. 6 - In writing thermochemical equations, why is it...Ch. 6 - Explain the meaning of this thermochemical...Ch. 6 - Consider this reaction:...Ch. 6 - The first step in the industrial recovery of zinc...Ch. 6 - Determine the amount of heat (in kJ) given off...Ch. 6 - Consider the reaction...Ch. 6 - Consider the reaction...Ch. 6 - What is the difference between specific heat and...Ch. 6 - Define calorimetry and describe two commonly used...Ch. 6 - Consider the following data: Metal Al Cu Mass (g)...Ch. 6 - A piece of silver of mass 362 g has a heat...Ch. 6 - A 6.22-kg piece of copper metal is heated from...Ch. 6 - Calculate the amount of heat liberated (in kJ)...Ch. 6 - A sheet of gold weighing 10.0 g and at a...Ch. 6 - To a sample of water at 23.4C in a...Ch. 6 - A 0.1375-g sample of solid magnesium is burned in...Ch. 6 - A quantity of 85.0 mL of 0.900 M HCl is mixed with...Ch. 6 - What is meant by the standard-state condition?Ch. 6 - How are the standard enthalpies of an element and...Ch. 6 - What is meant by the standard enthalpy of a...Ch. 6 - Write the equation for calculating the enthalpy of...Ch. 6 - State Hesss law. Explain, with one example, the...Ch. 6 - Describe how chemists use Hesss law to determine...Ch. 6 - Which of the following standard enthalpy of...Ch. 6 - The Hfo values of the two allotropes of oxygen, O2...Ch. 6 - Which is the more negative quantity at 25C: Hfo...Ch. 6 - Predict the value of Hfo (greater than, less than,...Ch. 6 - In general, compounds with negative Hfo values are...Ch. 6 - Suggest ways (with appropriate equations) that...Ch. 6 - Calculate the heat of decomposition for this...Ch. 6 - The standard enthalpies of formation of ions in...Ch. 6 - Calculate the heats of combustion for the...Ch. 6 - Calculate the heats of combustion for the...Ch. 6 - Methanol, ethanol, and n-propanol are three common...Ch. 6 - The standard enthalpy change for the following...Ch. 6 - From the standard enthalpies of formation,...Ch. 6 - Pentaborane-9, B5H9, is a colorless, highly...Ch. 6 - Determine the amount of heat (in kJ) given off...Ch. 6 - At 850C, CaCO3 undergoes substantial decomposition...Ch. 6 - From these data,...Ch. 6 - From the following data,...Ch. 6 - From the following heats of combustion,...Ch. 6 - Calculate the standard enthalpy change for the...Ch. 6 - Prob. 6.65QPCh. 6 - Why is the lattice energy of a solid always a...Ch. 6 - Consider two ionic compounds A and B. A has a...Ch. 6 - Mg2+ is a smaller cation than Na+ and also carries...Ch. 6 - Why is it dangerous to add water to a concentrated...Ch. 6 - Which of the following does not have Hfo=O at 25C?...Ch. 6 - Calculate the expansion work done when 3.70 moles...Ch. 6 - Prob. 6.73QPCh. 6 - Given the thermochemical equations:...Ch. 6 - The standard enthalpy change H for the thermal...Ch. 6 - Hydrazine, N2H4, decomposes according to the...Ch. 6 - A quantity of 2.00 102 mL of 0.862 M HCl is mixed...Ch. 6 - A 3.53-g sample of ammonium nitrate (NH4NO3) was...Ch. 6 - Consider the reaction...Ch. 6 - Prob. 6.80QPCh. 6 - Prob. 6.81QPCh. 6 - A 2.10-mole sample of crystalline acetic acid,...Ch. 6 - Prob. 6.83QPCh. 6 - You are given the following data:...Ch. 6 - A gaseous mixture consists of 28.4 mole percent of...Ch. 6 - When 2.740 g of Ba reacts with O2 at 298 K and 1...Ch. 6 - Methanol (CH3OH) is an organic solvent and is also...Ch. 6 - A 44.0-g sample of an unknown metal at 99.0C was...Ch. 6 - Using the data in Appendix 2, calculate the...Ch. 6 - Producer gas (carbon monoxide) is prepared by...Ch. 6 - Prob. 6.91QPCh. 6 - Prob. 6.92QPCh. 6 - Ethanol (C2H5OH) and gasoline (assumed to be all...Ch. 6 - The combustion of what volume of ethane (C2H6),...Ch. 6 - Prob. 6.95QPCh. 6 - Prob. 6.96QPCh. 6 - Explain the cooling effect experienced when...Ch. 6 - For which of the following reactions does...Ch. 6 - Prob. 6.99QPCh. 6 - A quantity of 0.020 mole of a gas initially at...Ch. 6 - Prob. 6.101QPCh. 6 - Prob. 6.102QPCh. 6 - Prob. 6.103QPCh. 6 - Prob. 6.104QPCh. 6 - A person ate 0.50 pound of cheese (an energy...Ch. 6 - Prob. 6.106QPCh. 6 - Prob. 6.107QPCh. 6 - The enthalpy of combustion of benzoic acid...Ch. 6 - Prob. 6.109QPCh. 6 - Prob. 6.110QPCh. 6 - Glaubers salt, sodium sulfate decahydrate (Na2SO4 ...Ch. 6 - A balloon 16 m in diameter is inflated with helium...Ch. 6 - Acetylene (C2H2) can be hydrogenated (reacting...Ch. 6 - Prob. 6.114QPCh. 6 - An excess of zinc metal is added to 50.0 mL of a...Ch. 6 - (a) A person drinks four glasses of cold water...Ch. 6 - Prob. 6.118QPCh. 6 - Why are cold, damp air and hot, humid air more...Ch. 6 - Prob. 6.120QPCh. 6 - Prob. 6.121QPCh. 6 - Prob. 6.122QPCh. 6 - Prob. 6.123QPCh. 6 - Determine the standard enthalpy of formation of...Ch. 6 - Prob. 6.125QPCh. 6 - Ice at 0C is placed in a Styrofoam cup containing...Ch. 6 - Prob. 6.127QPCh. 6 - Prob. 6.128QPCh. 6 - Calculate the internal energy of a Goodyear blimp...Ch. 6 - Prob. 6.131QPCh. 6 - Acetylene (C2H2) can be made by reacting calcium...Ch. 6 - The average temperature in deserts is high during...Ch. 6 - From a thermochemical point of view, explain why a...Ch. 6 - Calculate the U for the following reaction at 298...Ch. 6 - Lime is a term that includes calcium oxide (CaO,...Ch. 6 - A 4.117-g impure sample of glucose (C6H12O6) was...Ch. 6 - Construct a table with the headings q, w, U, and...Ch. 6 - The combustion of 0.4196 g of a hydrocarbon...Ch. 6 - Metabolic activity in the human body releases...Ch. 6 - Give an example for each of the following...Ch. 6 - From the following data, calculate the heat of...Ch. 6 - Starting at A, an ideal gas undergoes a cyclic...Ch. 6 - For reactions in condensed phases (liquids and...Ch. 6 - The diagrams (a)(d) represent various physical and...Ch. 6 - A 20.3-g sample of an unknown metal and a 28.5-g...Ch. 6 - Prob. 6.148QPCh. 6 - Prob. 6.149QPCh. 6 - The fastest serve in tennis is about 150 mph. Can...Ch. 6 - Prob. 6.151QPCh. 6 - It has been estimated that 3 trillion standard...Ch. 6 - Prob. 6.153QPCh. 6 - Prob. 6.154QPCh. 6 - Prob. 6.155QPCh. 6 - We hear a lot about how the burning of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
The validity of a scientific law.
Physical Universe
Give the IUPAC name for each compound.
Organic Chemistry
Describe the evolution of mammals, tracing their synapsid lineage from early amniote ancestors to true mammals....
Loose Leaf For Integrated Principles Of Zoology
On what molecule does the anticodon appear? Explain the role of this molecule in protein synthesis.
Human Physiology: An Integrated Approach (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forwardIdentify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forward
- Rank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forward
- Why isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forward
- Complete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forwardQ Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY