
Review of Selected Concepts Related to Nomenclature
Write the chemical formula of each of the following. The blue spheres represent nitrogen atoms and the red spheres oxygen atoms. Oxygen is written last in the formulas that include oxygen.
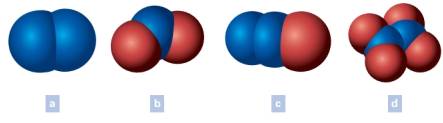
(a)
Interpretation:
The chemical formula of each of the following is to be written. The blue spheres represent nitrogen atoms and the red spheres oxygen atoms. Oxygen is written last in the formulas that include oxygen.
Concept introduction:
The particle of an element or compound in the written format is denoted by the chemical formulas. The symbols of elements in a particular substance covers the formula of the required substance.
Answer to Problem 1E
The chemical formula of (a) is written as N2
Explanation of Solution
Generally, in a formula, the total number of atoms of the element under study is shown by a subscript number immediately following the symbol. Notably, the subscript does not have the number when only one atom of an element present in the formula. The number of each kind of atom which makes up the particle generally called as the composition, is denoted by the chemical formula. Commonly, elements can be madeup of molecules having single atom, two atoms or complex multi-atoms. Coversely, when the substance itself is an element it should have all atoms of the same element. In the above example (a), the two lobes are having the same blue color which is for the element nitrogen.
Total number of lobes of nitrogen (N) = 2
Chemical formula = N2
Thus, the chemical formula of the colored lobes (a) is written.
(b)
Interpretation:
The chemical formula of each of the following is to be written. The blue spheres represent nitrogen atoms and the red spheres oxygen atoms. Oxygen is written last in the formulas that include oxygen.
Concept introduction:
The particle of an element or compound in the written format is denoted by the chemical formulas. The symbols of elements in a particular substance covers the formula of the required substance.
Answer to Problem 1E
The chemical formula of (b) is written as NO2
Explanation of Solution
Generally, in a formula, the total number of atoms of the element under study is shown by a subscript number immediately following the symbol. Notably, the subscript does not have the number when only one atom of an element present in the formula. In the above example (b), the two lobes are having the same color which is for the element oxygen and one lobe is having the color for nitrogen.
Total number of lobes of nitrogen (N) = 1
Total number of lobes of oxygen (O) = 2
Chemical formula = NO2
Thus, the chemical formula of the colored lobes (b) is written.
(c)
Interpretation:
The chemical formula of each of the following is to be written. The blue spheres represent nitrogen atoms and the red spheres oxygen atoms. Oxygen is written last in the formulas that include oxygen.
Concept introduction:
The particle of an element or compound in the written format is denoted by the chemical formulas. The symbols of elements in a particular substance covers the formula of the required substance.
Answer to Problem 1E
The chemical formula of (c) is written as N2O
Explanation of Solution
Generally, in a formula, the total number of atoms of the element under study is shown by a subscript number immediately following the symbol. Notably, the subscript does not have the number when only one atom of an element present in the formula. In the above example (c), the two lobes are having the same color which is for the element nitrogen and one lobe is having the color for oxygen.
Total number of lobes of nitrogen (N) = 2
Total number of lobes of oxygen (O) = 1
Chemical formula = N2O
Thus, the chemical formula of the colored lobes (c) is written.
(d)
Interpretation:
The chemical formula of each of the following is to be written. The blue spheres represent nitrogen atoms and the red spheres oxygen atoms. Oxygen is written last in the formulas that include oxygen.
Concept introduction:
The particle of an element or compound in the written format is denoted by the chemical formulas. The symbols of elements in a particular substance covers the formula of the required substance.
Answer to Problem 1E
The chemical formula of (d) is written as N2O4
Explanation of Solution
Generally, in a formula, the total number of atoms of the element under study is shown by a subscript number immediately following the symbol. Notably, the subscript does not have the number when only one atom of an element present in the formula. In the above example (d), the two lobes are having the same color which is for the element nitrogen and other four lobes are having the color for oxygen.
Total number of lobes of nitrogen (N) = 2
Total number of lobes of oxygen (O) = 4
Chemical formula = N2O4
Thus, the chemical formula of the colored lobes (d) is written.
Want to see more full solutions like this?
Chapter 6 Solutions
EBK INTRODUCTORY CHEMISTRY: AN ACTIVE L
- JON Determine the bund energy for UCI (in kJ/mol Hcl) using me balanced chemical equation and bund energies listed? का (My (9) +36/2(g)-(((3(g) + 3(g) A Hryn = -330. KJ bond energy и-н 432 bond bond C-1413 C=C 839 N-H 391 C=O 1010 S-H 363 б-н 467 02 498 N-N 160 N=N 243 418 C-C 341 C-0 358 C=C C-C 339 N-Br 243 Br-Br C-Br 274 193 614 (-1 214||(=olin (02) 799 C=N 615 AALarrow_forwardDetermine the bond energy for HCI ( in kJ/mol HCI) using he balanced cremiculequecticnand bund energles listed? also c double bond to N is 615, read numbets carefully please!!!! Determine the bund energy for UCI (in kJ/mol cl) using me balanced chemical equation and bund energies listed? 51 (My (9) +312(g)-73(g) + 3(g) =-330. KJ спод bond energy Hryn H-H bond band 432 C-1 413 C=C 839 NH 391 C=O 1010 S-1 343 6-H 02 498 N-N 160 467 N=N C-C 341 CL- 243 418 339 N-Br 243 C-O 358 Br-Br C=C C-Br 274 193 614 (-1 216 (=olin (02) 799 C=N 618arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- I need help on my practice final, if you could explain how to solve this that would be extremely helpful for my final thursday. Please dumb it down chemistry is not my strong suit. If you could offer strategies as well to make my life easier that would be beneficialarrow_forwardNonearrow_forwardNonearrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





