
CAMPBEL BIOLOGY:CONCEPTS & CONNECTIONS
10th Edition
ISBN: 9780136538820
Author: Taylor
Publisher: INTER PEAR
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 15TYK
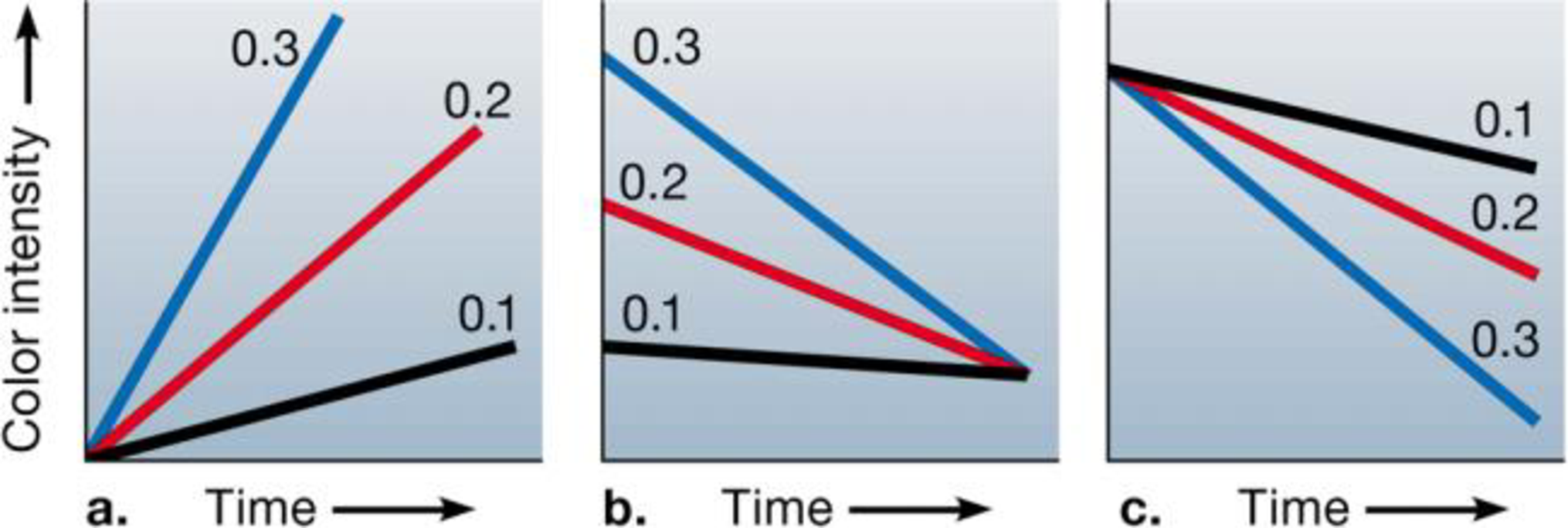
In the citric acid cycle, an enzyme oxidizes malate to oxaloacetate, with the production of NADH and the release of H+. You are studying this reaction using a suspension of bean cell mitochondria and a blue dye that loses its color as it takes up H+. You set up reaction mixtures with mitochondria, dye, and three different concentrations of malate (0.1 mg/L, 0.2 mg/L, and 0.3 mg/L). Which of the following graphs represents the results you would expect, and why?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
You are studying the effect of directional selection on body height in three
populations (graphs a, b, and c below).
(a) What is the selection differential? Show your calculation. (2 pts)
(b) Which population has the highest narrow sense heritability for height?
Explain your answer. (2 pts)
(c) If you examined the offspring in the next generation in each population,
which population would have the highest mean height? Why? (2 pts)
(a)
Midoffspring height (average height of offspring)
Short
Short
Short
Short
(c)
Short
(b)
Short
Tall
Short
Tall
Short
Short
Tall
Midparent height
(average height of
Mean of population = 65 inches
Mean of breading parents = 70 inches
Mean of population = 65 inches
Mean of breading parents = 70 inches
Mean of population = 65 inches
Mean of breading parents = 70 inches
P
You are studying a population of 100 flowers that has two alleles at a locus for
flower color, blue (B) and green (G). There are 15 individuals with the BB
genotype, 70 individuals with the BG genotype, and 15 individuals with the GG
genotype.
(a) What are the allele frequencies of B and G in the starting population? Show
your calculations. (2 pts)
(b) Is this population in Hardy-Weinberg equilibrium? Show your calculations. (3
pts)
12pt v
Paragraph
BIU UA
AV & VT2V
f
CO
V
In a natural population of outbreeding plants, the variance of the total number
of seeds per plant is 16. From the natural population, 20 plants are taken into
the laboratory and developed into separate true-breeding lines by self-
fertilization-with selection for high, low, or medium number of seeds-for 10
generations. The average variance in the tenth generation in each of the 20 sets
is about equal and averages 5.8 across all the sets. Estimate the broad-sense
heritability for seed number in this population. (4 pts)
12pt v
Paragraph
BIUA V
V
T² v
B
①
O words
Chapter 6 Solutions
CAMPBEL BIOLOGY:CONCEPTS & CONNECTIONS
Ch. 6 - Fill in the blanks in this summary map to help you...Ch. 6 - A biochemist wanted to study how various...Ch. 6 - In glycolysis, _____ is oxidized and _____ is...Ch. 6 - Most of the CO2 from cellular respiration is...Ch. 6 - Which of the following is the most immediate...Ch. 6 - Which of the following is a true distinction...Ch. 6 - The poison cyanide binds to an electron carrier...Ch. 6 - In which of the following is the first molecule...Ch. 6 - Which of the three stages of cellular respiration...Ch. 6 - Compare and contrast fermentation as it occurs in...
Ch. 6 - Prob. 11TYKCh. 6 - Prob. 12TYKCh. 6 - Prob. 13TYKCh. 6 - Oxidative phosphorylation involves the flow of...Ch. 6 - In the citric acid cycle, an enzyme oxidizes...Ch. 6 - ATP synthase enzymes are found in the prokaryotic...Ch. 6 - Prob. 17TYKCh. 6 - For a short time in the 1930s, some physicians...Ch. 6 - Explain how the mechanism of brown fat metabolism...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- In a natural population of outbreeding plants, the variance of the total number of seeds per plant is 16. From the natural population, 20 plants are taken into the laboratory and developed into separate true-breeding lines by self- fertilization-with selection for high, low, or medium number of seeds-for 10 generations. The average variance in the tenth generation in each of the 20 sets is about equal and averages 5.8 across all the sets. Estimate the broad-sense heritability for seed number in this population. (4 pts) 12pt v Paragraph BI DI T² v ✓ B°arrow_forwardQuestion 1 In a population of Jackalopes (pictured below), horn length will vary between 0.5 and 2 feet, with the mean length somewhere around 1.05 feet. You pick Jackalopes that have horn lengths around 1.75 feet to breed as this appears to be the optimal length for battling other Jackalopes for food. After a round of breeding, you measure the offsprings' mean horn length is 1.67. What is the heritability of horns length (h2)? Is Jackalope horn length a heritable trait? (4 pts)? 12pt v Paragraph BIU A ✓arrow_forwardFrequency of allele A1 Question 2 The graph below shows results of two simulations, both depicting the rise in frequency of beneficial allele in a population of infinite size. The selection coefficient and the starting frequency are the same, but in one simulation the beneficial allele is dominant and in the other it is recessive. Neither allele is fixed by 500 generations. 1.0 1 0.8 0.6 0.4 2 0.2 0 0 100 200 300 400 500 Generation (a) Which simulation shows results for a dominant and which shows results for a recessive allele? How can you tell? (4 pts) (b) Neither of the alleles reaches fixation by 500 generations. If given enough time, will both of these alleles reach fixation in the population? Why or why not? (3 pts) 12pt Paragraph BIU AT2v Varrow_forward
- Question 14 The relative fitnesses of three genotypes are WA/A= 1.0, WA/a = 0.7, and Wa/a = 0.3. If the population starts at the allele frequency p = 0.5, what is the value of p in the next generation? (3 pts) 12pt v V Paragraph B I U D V T² v V V p O words <arrow_forwardAccording to a recent study, 1 out of 50,000 people will be diagnosed with cystic fibrosis. Cystic fibrosis can be caused by a mutant form of the CFTR gene (dominant gene symbol is F and mutant is f). A. Using the rate of incidence above, what is the frequency of carriers of the cystic fibrosis allele for CFTR in the US? (3 pts) B. In a clinical study, 400 people from the population mentioned in (A.) were genotyped for BRCA1 Listed below are the results. Are these results in Hardy- Weinberg equilibrium? Use Chi Square to show whether or not they are. (3 pts) BRCA1 genotype # of women 390 BB Bb bb 10 0 12pt Paragraph L BIUAV V T² v Varrow_forwardOutline a method for using apomixis to maintain feminized CannabisAssume apomixis is controlled by a single dominant gene. You can choose the type of apomixis: obligate or facultative, gametophytic or sporophytic. Discuss advantages and disadvantages of your proposed method.arrow_forward
- Kinetics: One-Compartment First-Order Absorption 1. In vivo testing provides valuable insight into a drug’s kinetics. Assessing drug kinetics following multiple routes of administration provides greater insight than a single route of administration alone. The following data was collected in 250-g rats following bolus IV, oral (PO), and intraperitoneal (ip) administration. Using this data and set of graphs, determine:(calculate for each variable) (a) k, C0, V, and AUC* for the bolus iv data (b) k, ka, B1, and AUC* for the po data c) k, ka, B1, and AUC* for the ip data (d) relative bioavailability for po vs ip, Fpo/Fip (e)absolute ip bioavailability, Fip (f) absolute po bioavailability, Fpoarrow_forward3. A promising new drug is being evaluated in human trials. Based on preliminary human tests, this drug is most effective when plasma levels exceed 30 mg/L. Measurements from preliminary tests indicate the following human pharmacokinetic parameter values: t1/2,elim = 4.6hr, t1/2,abs = 0.34hr, VD = 0.29 L/kg, Foral = 72%. Based on these parameters, estimate the following if a 49 kg woman were to receive a 1000mg oral dose of this drug: (a) Estimate the plasma concentration of the drug at 1hr, 6 hr, and 20hr after taking the drug ( Concentration estimate) (b) Estimate the time for maximum plasma concentration (tmax). (c) Estimate the maximum plasma concentration (Cmax). (d) Estimate the time at which the plasma level first rises above 30 mg/L. (Note this is a trial and error problem where you must guess a time, plug it into the concentration equation, and determine if it is close to 30 mg/L. Hint: based on part (a) it should be apparent that the answer is less than 1hr.) (e)…arrow_forwardList substitutions in your diet you could make to improve it based on what you know now about a balanced diet. For instance, if you like to drink soda, you might substitute skim milk or water for some of the soft drinks you consumed. List the item you wish to replace with the new item and what you hope to accomplish with that substitution. Be sure to choose foods you know that you'd enjoy and you consider more "healthful." If you feel your diet is already balanced, describe how you accomplish your balanced intake and when you began eating this way.arrow_forward
- Which single food item contained you ate for the 3 days with the most sodium?arrow_forwardSelect a diet and choose one site that provides credible information. Explain why the source itself and/or the information on the site is credible. This should be a report. This site should be different from U.S News and World Report. For full credit, you must include the following information and elaborate in detail: The diet name The main components of the diet The credible website name and link What makes the credible website credible?arrow_forwardSelect a diet and give a summary of the main components. Do a web search of the diet and choose one link that provides misinformation. Explain why the site itself and/or the information on the site is not credible. This should be a report. This site should be different from U.S News and World Report. For full credit, you must include the following information and elaborate in detail: The diet name The main components of the diet The misinformation website and link What misinformation is being provided in the other website and how did you determine it was not credible?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning

Human Physiology: From Cells to Systems (MindTap ...
Biology
ISBN:9781285866932
Author:Lauralee Sherwood
Publisher:Cengage Learning

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Concepts of Biology
Biology
ISBN:9781938168116
Author:Samantha Fowler, Rebecca Roush, James Wise
Publisher:OpenStax College

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Cengage Learning
Intro to Food Microbiology; Author: A professor pressing record;https://www.youtube.com/watch?v=vg8fSmk0dVU;License: Standard youtube license