
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5.9B, Problem 5.15P
Draw three-dimensional representations of the following compounds. Which have asymmetric carbon atoms'? Which have no asymmetric carbons but are chiral anyway? Use your models for parts (a) through (d) and any others that seem unclear.
- a. CIHC=C=CHCI 1,3dichioroprop8diene
- b. CIHC=C=CHCH3 1-chlorobuta-1,2-diene
- c. CIHC=C=C(CH3) 1-chloro-3-methy1buta-1,2-diene
- d. CIHC=CH—CH=CH2 1-chlorobuta-1,3-diene
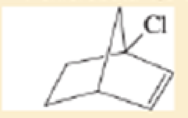
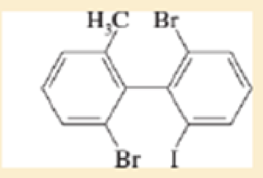
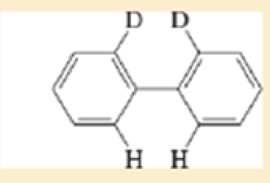
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
What is the final product when D-galactose reacts with hydroxylamine?
Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.
Chapter 5 Solutions
Organic Chemistry (9th Edition)
Ch. 5.2 - Determine whether the following objects are chiral...Ch. 5.2A - Prob. 5.2PCh. 5.2B - Prob. 5.3PCh. 5.2B - Prob. 5.4PCh. 5.2C - Prob. 5.5PCh. 5.3 - Prob. 5.6PCh. 5.3 - Prob. 5.7PCh. 5.4D - Prob. 5.8PCh. 5.4D - Prob. 5.9PCh. 5.4D - Prob. 5.10P
Ch. 5.5 - Prob. 5.11PCh. 5.7 - When optically pure (R)-2-bromobutane is heated...Ch. 5.7 - Prob. 5.13PCh. 5.8 - Prob. 5.14PCh. 5.9B - Draw three-dimensional representations of the...Ch. 5.10A - For each sot of examples, make a model of the...Ch. 5.10A - Draw a Fischer projection for each compound....Ch. 5.10B - Prob. 5.18PCh. 5.10C - For each Fischer projection, label each asymmetric...Ch. 5.11C - Prob. 5.20PCh. 5.13 - Prob. 5.21PCh. 5.13 - Prob. 5.22PCh. 5.15 - Prob. 5.23PCh. 5.16A - Prob. 5.24PCh. 5 - The following four structures are naturally...Ch. 5 - For each structure, 1. star () any asymmetric...Ch. 5 - Prob. 5.27SPCh. 5 - Prob. 5.28SPCh. 5 - Prob. 5.29SPCh. 5 - Prob. 5.30SPCh. 5 - Prob. 5.31SPCh. 5 - Prob. 5.32SPCh. 5 - Prob. 5.33SPCh. 5 - Prob. 5.34SPCh. 5 - For each structure, 1. draw all the stereoisomers....Ch. 5 - Prob. 5.36SPCh. 5 - Prob. 5.37SPCh. 5 - 3,4-Dimethylpent-1-ene has the formula...Ch. 5 - A graduate student was studying enzymatic...Ch. 5 - Prob. 5.40SPCh. 5 - Prob. 5.41SP
Additional Science Textbook Solutions
Find more solutions based on key concepts
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
Describe the evolution of mammals, tracing their synapsid lineage from early amniote ancestors to true mammals....
Loose Leaf For Integrated Principles Of Zoology
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License