
(a)
Interpretation: The acid base reaction of aniline with hydrochloric acid needs to be explained.
Concept Introduction:Bronsted and Lowery purposed the Bronsted-Lowry acid-base theory. It states that acid can give
(b)
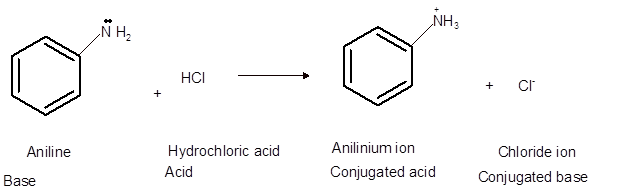
Interpretation: The acid, base, conjugated acid and conjugated base in the reaction of aniline with hydrochloric acid needs to be determined.
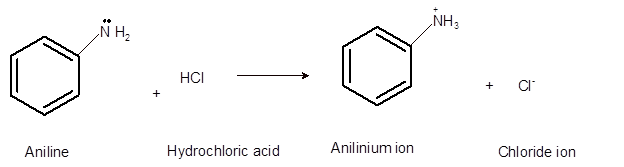
Concept Introduction:Bronsted and Lowery purposed the Bronsted-Lowry acid-base theory. It states that acid can give
(c)
Interpretation: The solubility of aniline and its conjugated base in diethyl ether and water needs to be explained.
Concept Introduction:Bronsted and Lowery purposed the Bronsted-Lowry acid-base theory. It states that acid can give
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
- Below is the SN1 reaction of (S)-3-chlorocyclohexene and hydroxide ("OH). Draw the missing curved arrows, lone pairs of electrons, and nonzero formal charges. In the third box, draw the two enantiomeric products that will be produced. 2nd attempt Please draw all four bonds at chiral centers. 0 D Draw the missing curved arrow notation. Add lone pairs of electrons and nonzero formal charges. + 노 V 1st attempt Feedback Please draw all four bonds at chiral centers. See Periodic Table See Hint F P 41 H Br See Periodic Table See Hint H Larrow_forwardHow close are the Mulliken and Pauling electronegativity scales? (a) Now that the ionization energies and electron affinities have been defined, calculate the Mulliken and Pauling electronegativities for C, N, O and F. Compare them. (Make the necessary adjustments to the values, such as dividing the ionization energies and electron affinities by 230kj/mol) (b) Plot both sets of electronegativities against atomic number (use the same graph). (c) Which scale depends most consistently on position in the Periodic Table?arrow_forwardBelow is the SN2 reaction between 2-bromopropane and iodide (I). Draw the mechanism arrows in the first box to reflect electron movements. In both boxes, add lone pairs of electrons and nonzero formal charges. 4th attempt Feedback 3rd attempt Feedback 1 -Br H :Bri :Br: ili See Periodic Table See Hint ini See Periodic Table See Hintarrow_forward
- When 4-chloro-1-butanol is placed in sodium hydride, a cyclization reaction occurs. 3rd attempt 2 HO NaH CI D Draw the curved arrow notation to form the intermediate. 4 2 H₂ See Periodic Table See Hint =arrow_forwardSketch, qualitatively, the potential energy curves of the N-N bond of N2H4, N2 and N3- graph. Explain why the energy at the minimum of each curve is not the same.arrow_forward(a) Show that the lattice energies are inversely proportional to the distance between ions in MX (M = alkali metal, X = halide ions) by plotting the lattice energies of KF, KCl, and KI against the internuclear distances, dMX. The lattice energies of KF, KCl, and KI are 826, 717, and 645 kJ/mol, respectively. Does the correlation obtained correlate well? You will need to use a standard graphing program to construct the graph (such as a spreadsheet program). It will generate an equation for the line and calculate a correlation coefficient. (b) Estimate the lattice energy of KBr from your graph. (c) Find an experimental value for the lattice energy of KBr in the literature, and compare this value with the one calculated in (b). Do they agree?arrow_forward
- Show the curved arrow mechanism and both products for the reaction between methyl iodide and propoxide. 1st attempt NV H 10: H H 1 Add the missing curved arrow notation. H + See Periodic Tablearrow_forwardFirst I wanted to see if you would mind checking my graphs behind me. (They haven't been coming out right)? Second, could you help me explain if the rate of reaction is proportional to iodide and persulfate of each graph. I highlighted my answer and understanding but I'm not sure if I'm on the right track. Thank you in advance.arrow_forwardThe heat of combustion for ethane, C2H6C2H6 , is 47.8 kJ/g. How much heat is produced if 1.65 moles of ethane undergo complete combustion?arrow_forward
- Review of this week's reaction: H2NCN (cyanamide) + CH3NHCH2COOH (sarcosine) + NaCl, NH4OH, H2O ----> H2NC(=NH)N(CH3)CH2COOH (creatine) Q7. Draw by hand the reaction of creatine synthesis listed above using line structures without showing the Cs and some of the Hs, but include the lone pairs of electrons wherever they apply. (4 pts) Q8. Considering the Zwitterion form of an amino acid, draw the Zwitterion form of Creatine. (2 pts) Q9. Explain with drawing why the C—N bond shown in creatine structure below can or cannot rotate. (3 pts)arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forwardPlease help me answer a. Please and thank you I advance.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

