
Concept explainers
(a)
Interpretation: The elements with the given electronic configuration should be identified as a metal, non-metal or metalloid.
Concept Introduction: The elements which can donate electrons to get the stable electronic configuration are metal, elements that can accept electrons are non-metal. The elements with characteristics of both metals and non-metals are metalloids.
(b)
Interpretation: The element with the smallest atomic size should be identified.
Concept Introduction: According to the periodic trends, the atomic size of the element increases on moving top to bottom in a group due to addition of one shell and decreases on moving left to right in a group due to increase in
(c)
Interpretation: The element with the highest ionization energy should be identified.
Concept Introduction: Ionization energy is defined as the amount of energy required to remove an electron from its outermost shell. According to periodic trends, it decreases on moving top to bottom in a group due to increase in size and it increases on moving left to right in a period due to decrease in size.
(d)
Interpretation: The element with the half-filled sublevel should be identified.
Concept Introduction: The relative energy of orbitals is represented as follows:
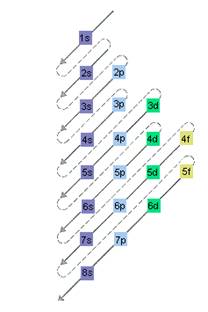
A s orbital can have maximum of 2 electrons, p orbital can have maximum of 6 electrons. Similarly, maximum electrons that a d and f orbital can have are 10 and 14 respectively.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
EP BASIC CHEMISTRY-MODIFIED MASTERING
- Identify the missing organic reactants in the following reaction: X + Y H+ two steps Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule reactants or products (like H2O) are not shown. In the drawing area below, draw the skeletal ("line") structures of the missing organic reactants X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. Х :arrow_forwardDraw the mechanism of friedel-crafts acylation using acetyl chloride of m-Xylenearrow_forwardI need help naming these in IUPACarrow_forward
- H R Part: 1/2 :CI: is a/an electrophile Part 2 of 2 Draw the skeletal structure of the product(s) for the Lewis acid-base reaction. Include lone pairs and formal charges (if applicable) on the structures. 4-7: H ö- H Skip Part Check X :C1: $ % L Fi Click and drag to start drawing a structure. MacBook Pro & ㅁ x G 0: P Add or increase positive formal cha Save For Later Submit ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forwardDraw the friedel-crafts acylation mechanism of m-Xylenearrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- 1. Base on this experimental results, how do you know that the product which you are turning in is methyl 3-nitrobenzoate(meta substituted product ) rather than either of the other two products? 2. What observation suggests that at least a small amount of one or both of the other two isomers are in the mother liquor?arrow_forwardExplain Huckel's rule.arrow_forwardhere is my question can u help me please!arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





