
MOLECULAR NATURE OF MATTER 7/E LL W/AC
7th Edition
ISBN: 9781119664796
Author: JESPERSEN
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 73RQ
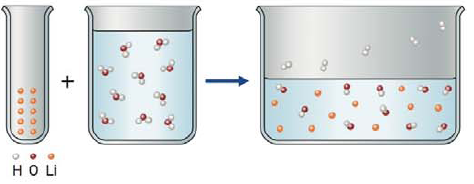
Write the balanced chemical equation for the following reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
EEZE
LETCHUP
ID
Draw the most likely conjugate base resulting from this acid-base reaction.
Include all lone pairs. Ignore inorganic byproducts.
Drawing
く
NaOCH2CH3
:0:
:0:
狗
Answer
2. Provide a clear arrow-pushing mechanism for the following reactions. Do not skip proton
transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted
without ambiguity.
a.
CH3
Ph
OEt
هد
Ph
CH3
Hint: the species on the left is an ynolate, which behaves a lot like an enolate.
Chapter 5 Solutions
MOLECULAR NATURE OF MATTER 7/E LL W/AC
Ch. 5 - When sodium reacts with molecular oxygen, O2, the...Ch. 5 - Prob. 2PECh. 5 - The chlorite ion, ClO2, is a potent disinfectant,...Ch. 5 - Assign oxidation numbers to each atom in...Ch. 5 - Prob. 5PECh. 5 - Chlorine dioxide, ClO2, is used to kill bacteria...Ch. 5 - The following equation is nor balanced. Explain...Ch. 5 - Practice Exercise 5.8
The element technetium...Ch. 5 - Practice Exercise 5.9
Consider the following...Ch. 5 - Balance the following equation for a basic...
Ch. 5 - Write the balanced half-react ions for the...Ch. 5 - Prob. 12PECh. 5 - Suppose an aqueous mixture is prepared containing...Ch. 5 - Prob. 14PECh. 5 - Prob. 15PECh. 5 - Write a balanced equation for the combustion of...Ch. 5 - Prob. 17PECh. 5 - Prob. 18PECh. 5 - Prob. 1RQCh. 5 - Prob. 2RQCh. 5 - Prob. 3RQCh. 5 - Are the following redox reactions? Explain....Ch. 5 - If the oxidation number of nitrogen in a certain...Ch. 5 - When balancing redox reactions, which side of a...Ch. 5 - Prob. 7RQCh. 5 - What are the net charges on the left and right...Ch. 5 - In Question 5.8, which half-reaction represents...Ch. 5 - The following equation is not balanced....Ch. 5 - 5.11 What is a nonoxidizing acid? Give two...Ch. 5 - What is the strongest oxidizing agent in an...Ch. 5 - Prob. 13RQCh. 5 - Prob. 14RQCh. 5 - What is a single replacement reaction?Ch. 5 - If a metal is able to react with a solution of...Ch. 5 - Prob. 17RQCh. 5 - Prob. 18RQCh. 5 - Where in the activity series do we find the best...Ch. 5 - When manganese reacts with silver ions, is...Ch. 5 - Prob. 21RQCh. 5 - Prob. 22RQCh. 5 - Prob. 23RQCh. 5 - If one of the impurities in diesel fuel has the...Ch. 5 - Prob. 25RQCh. 5 - Prob. 26RQCh. 5 - Prob. 27RQCh. 5 - Potassium permanganate is often used for redox...Ch. 5 - Assign oxidation numbers to the atoms in the...Ch. 5 - 5.30 Assign oxidation numbers to the atoms in the...Ch. 5 - Assign oxidation numbers to each atom in the...Ch. 5 - Assign oxidation numbers to the elements...Ch. 5 - 5.33 Assign oxidation numbers to the elements in...Ch. 5 - 5.34 Assign oxidation numbers to the elements in...Ch. 5 - Titanium burns in pure nitrogen to form TiN. What...Ch. 5 - 5.36 Zirconia, which is , is used to make ceramic...Ch. 5 - 5.37 Ozone, , is an allotrope of oxygen and is one...Ch. 5 - 5.38 The other major air pollutant is . What are...Ch. 5 - For the following reactions, identify the...Ch. 5 - 5.40 For the following reactions, identify the...Ch. 5 - When chlorine is added to drinking water to kill...Ch. 5 - One pollutant in smog is nitrogen dioxide. NO2....Ch. 5 - Balance the following equations for reactions...Ch. 5 - 5.44 Balance the following equations for reactions...Ch. 5 - Prob. 45RQCh. 5 - 5.46 Balance the following equations for reactions...Ch. 5 - 5.47 Balance the equations for the following...Ch. 5 - Balance the equations for the following reactions...Ch. 5 - Prob. 49RQCh. 5 - 5.50 Hydroiodic acid reduces chlorine to...Ch. 5 - Prob. 51RQCh. 5 - Corn is grown for human consumption, feeding...Ch. 5 - Prob. 53RQCh. 5 - Laundry bleach such as Clorox is a dilute solution...Ch. 5 - Prob. 55RQCh. 5 - Chlorine is a good bleaching agent because it is...Ch. 5 - Write balanced molecular, ionic, and net ionic...Ch. 5 - 5.58 Write balanced molecular, ionic, and net...Ch. 5 - Prob. 59RQCh. 5 - Prob. 60RQCh. 5 - Prob. 61RQCh. 5 - Prob. 62RQCh. 5 - Prob. 63RQCh. 5 - Hot, concentrated sulfuric acid is a fairly strong...Ch. 5 - Use Table 5.3 to predict the outcome of the...Ch. 5 - 5.66 Use Table 5.3 to predict the outcome of the...Ch. 5 - The following reactions occur spontaneously....Ch. 5 - The following reactions occur spontaneously....Ch. 5 - Prob. 69RQCh. 5 - When chromium metal is dipped into a solution of...Ch. 5 - Write a balanced equation for the reaction...Ch. 5 - Write a balanced equation for the reaction...Ch. 5 - Write the balanced chemical equation for the...Ch. 5 - Write the balanced chemical equation for the...Ch. 5 - 5.75 Write balanced chemical equations for the...Ch. 5 - Write balanced chemical equations for the complete...Ch. 5 - 5.77 Write balanced equations for the combustion...Ch. 5 - 5.78 Write balanced equations for the combustion...Ch. 5 - 5.79 The metabolism of carbohydrates such as...Ch. 5 - Methanol, CH3OH, has been suggested as an...Ch. 5 - Write the balanced equation for the combustion of...Ch. 5 - 5.82 Thiophene, , is an impurity in crude oil and...Ch. 5 - Write chemical equations for the reaction of...Ch. 5 - Prob. 84RQCh. 5 - Prob. 85RQCh. 5 - Prob. 86RQCh. 5 - In an acidic solution, permanganate ion reacts...Ch. 5 - 5.88 In an acidic solution, bisulfite ion reacts...Ch. 5 - Prob. 89RQCh. 5 - Potable water (drinking water) should not have...Ch. 5 - 5.91 Sulfites are used worldwide in the wine...Ch. 5 - Methylbromide, CH3Br, is used in agriculture to...Ch. 5 - A sample of a copper ore with a mass of 0.4225 g...Ch. 5 - 5.94 A 1.362 g sample of an iron ore that...Ch. 5 - 5.95 Hydrogen peroxide, , solution can be...Ch. 5 - 5.96 Sodium nitrite, , is used as a preservative...Ch. 5 - Prob. 97RQCh. 5 - Prob. 98RQCh. 5 - Both calcium chloride and sodium chloride are used...Ch. 5 - 5.100 One way to analyze a sample for nitrite ion...Ch. 5 - Use oxidation numbers to show that the...Ch. 5 - Prob. 102RQCh. 5 - Prob. 103RQCh. 5 - Prob. 104RQCh. 5 - Balance the following equations using the...Ch. 5 - Prob. 106RQCh. 5 - What is the average oxidation number of carbon in...Ch. 5 - Prob. 108RQCh. 5 - In Problem 5-108, were all of the experiments...Ch. 5 - Prob. 110RQCh. 5 - 5.111 In each of the following pairs, choose the...Ch. 5 - Use Table 5.3 to predict whether the following...Ch. 5 - Sucrose, C12H22O11, is ordinary table sugar. Write...Ch. 5 - Balance the following equations by the...Ch. 5 - 5.115 Lead(IV) oxide reacts with hydrochloric acid...Ch. 5 - Prob. 116RQCh. 5 - A copper bar with a mass of 12.340 g is dipped...Ch. 5 - Prob. 118RQCh. 5 - 5.119 As described in the Chemistry Outside the...Ch. 5 - Titanium(IV) can be reduced to titanium(III) by...Ch. 5 - A researcher planned to use chlorine gas in an...Ch. 5 - 5.122 A sample of a tin ore weighing 0.3000 g was...Ch. 5 - Biodiesel is formed from the reaction of oils with...Ch. 5 - *5.124 In June 2002, the Department of Health and...Ch. 5 - An organic compound contains carbon, hydrogen, and...Ch. 5 - *5.126 A mixture is made by combining with ....Ch. 5 - A solution containing 0.1244 g of K2C2O4 was...Ch. 5 - It was found that a 20.0 mL portion of a solution...Ch. 5 - A 15.00 mL sample of a solution containing oxalic...Ch. 5 - A solution with a volume of 500.0 mL contained a...Ch. 5 - Prob. 131RQCh. 5 - We described the ion-electron method for balancing...Ch. 5 - Assuming that a chemical reaction with DNA could...Ch. 5 - Would you expect atomic oxygen and chlorine to be...Ch. 5 - Prob. 135RQCh. 5 - Prob. 136RQ
Additional Science Textbook Solutions
Find more solutions based on key concepts
Give your own example of how the structure of a part of the body is related to its function.
Principles of Anatomy and Physiology
Which one of the following is not a fuel produced by microorganisms? a. algal oil b. ethanol c. hydrogen d. met...
Microbiology: An Introduction
2. How many significant figures does each of the following numbers have?
a. 290 b. 2.90 × 104 c. 0.0029 d. 2.9...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Use the following graph to answer questions 3 and 4. 3. Which of the lines best depicts the log phase of a ther...
Microbiology: An Introduction
A simple magnifier of focal length f is placed near the eye of someone whose near point Pn is 25 cm. An object ...
Fundamentals of Physics Extended
Why are BSL-4 suits pressurized? Why not just wear tough regular suits?
Microbiology with Diseases by Body System (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- b. CH3 H3C CH3 CH3 H3C an unexpected product, containing a single 9- membered ring the expected product, containing two fused rings H3C-I (H3C)2CuLi an enolatearrow_forwardb. H3C CH3 1. 2. H3O+ H3C MgBr H3Carrow_forwardPredict the major products of this reaction: excess H+ NaOH ? A Note that the first reactant is used in excess, that is, there is much more of the first reactant than the second. If there won't be any products, just check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privarrow_forward
- 1. For each of the reaction "railroads" below, you are either asked to give the structure(s) of the starting material(s) or product(s), or provide reagents/conditions to accomplish the transformation, as indicated by the boxes. a. NaOMe H+ .CO,H HO₂C MeOH (excess) MeOH H3C Br يع CH3 1. LiAlH4 2. H3O+ 3. PBг3 H3C 1. Et-Li 2. H3O+ -CO₂Me -CO₂Me OH CH3 CH3 ল CH3arrow_forwardPredict the intermediate 1 and final product 2 of this organic reaction: NaOMe ག1, ད།་, - + H You can draw 1 and 2 in any arrangement you like. 2 work up Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds at the chiral center. Explanation Check Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Parrow_forwardWhat is the total energy cost associated with the compound below adopting the shown conformation? CH3 HH DH CH3arrow_forward
- ΗΝ, Draw Final Product C cyclohexanone pH 4-5 Edit Enamine H3O+ CH3CH2Br THF, reflux H Edit Iminium Ionarrow_forwardHow many hydrogen atoms are connected to the indicated carbon atom?arrow_forwardIdentify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forward
- Identify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning  Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY