
Concept explainers
(a)
Interpretation:
The electron dot structure for the atom of
Concept introduction:
The electron dot formula is used for the identification of valence electrons present in an atom. It shows the symbol of an atom with its valence electron around it. It is also called as Lewis dot structure. The valence electrons around an atom are shown by dots. One dot represents one valence electron.
The rules to draw electron dot formula are given below.
• First, the
• With the help of group number, the number of valence electrons is determined.
• The valance electrons are drawn around the atom by dots.
Answer to Problem 62E
The electron dot structure for the atom of
![]()
Explanation of Solution
The
![]()
Figure 1
The electron dot structure of
(b)
Interpretation:
The electron dot structure for the atom of
Concept introduction:
The electron dot formula is used for the identification of valence electrons present in an atom. It shows the symbol of an atom with its valence electron around it. It is also called as Lewis dot structure. The valence electrons around an atom are shown by dots. One dot represents one valence electron.
The rules to draw electron dot formula are given below.
• First, the symbol of an element is written.
• With the help of group number, the number of valence electrons is determined.
• The valance electrons are drawn around the atom by dots.
Answer to Problem 62E
The electron dot structure for the atom of
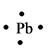
Explanation of Solution
Lead belongs to the group
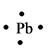
Figure 2
The electron dot structure of
(c)
Interpretation:
The electron dot structure for the atom of
Concept introduction:
The electron dot formula is used for the identification of valence electrons present in an atom. It shows the symbol of an atom with its valence electron around it. It is also called as Lewis dot structure. The valence electrons around an atom are shown by dots. One dot represents one valence electron.
The rules to draw electron dot formula are given below.
• First, the symbol of an element is written.
• With the help of group number, the number of valence electrons is determined.
• The valance electrons are drawn around the atom by dots.
Answer to Problem 62E
The electron dot structure for the atom of
![]()
Explanation of Solution
Selenium belongs to the group
![]()
Figure 3
The electron dot structure of
(d)
Interpretation:
The electron dot structure for the atom of
Concept introduction:
The electron dot formula is used for the identification of valence electrons present in an atom. It shows the symbol of an atom with its valence electron around it. It is also called as Lewis dot structure. The valence electrons around an atom are shown by dots. One dot represents one valence electron.
The rules to draw electron dot formula are given below.
• First, the symbol of an element is written.
• With the help of group number, the number of valence electrons is determined.
• The valance electrons are drawn around the atom by dots.
Answer to Problem 62E
The electron dot structure for the atom of
![]()
Explanation of Solution
Neon belongs to the group
![]()
Figure 4
The electron dot structure of
(e)
Interpretation:
The electron dot structure for the atom of
Concept introduction:
The electron dot formula is used for the identification of valence electrons present in an atom. It shows the symbol of an atom with its valence electron around it. It is also called as Lewis dot structure. The valence electrons around an atom are shown by dots. One dot represents one valence electron.
The rules to draw electron dot formula are given below.
• First, the symbol of an element is written.
• With the help of group number, the number of valence electrons is determined.
• The valance electrons are drawn around the atom by dots.
Answer to Problem 62E
The electron dot structure for the atom of
![]()
Explanation of Solution
Cesium belongs to the group
![]()
Figure 5
The electron dot structure of
(f)
Interpretation:
The electron dot structure for the atom of
Concept introduction:
The electron dot formula is used for the identification of valence electrons present in an atom. It shows the symbol of an atom with its valence electron around it. It is also called as Lewis dot structure. The valence electrons around an atom are shown by dots. One dot represents one valence electron.
The rules to draw electron dot formula are given below.
• First, the symbol of an element is written.
• With the help of group number, the number of valence electrons is determined.
• The valance electrons are drawn around the atom by dots.
Answer to Problem 62E
The electron dot structure for the atom of
![]()
Explanation of Solution
Gallium belongs to the group
![]()
Figure 6
The electron dot structure of
(g)
Interpretation:
The electron dot structure for the atom of
Concept introduction:
The electron dot formula is used for the identification of valence electrons present in an atom. It shows the symbol of an atom with its valence electron around it. It is also called as Lewis dot structure. The valence electrons around an atom are shown by dots. One dot represents one valence electron.
The rules to draw electron dot formula are given below.
• First, the symbol of an element is written.
• With the help of group number, the number of valence electrons is determined.
• The valance electrons are drawn around the atom by dots.
Answer to Problem 62E
The electron dot structure for the atom of
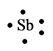
Explanation of Solution
Antimony belongs to the group
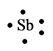
Figure 7
The electron dot structure of
(h)
Interpretation:
The electron dot structure for the atom of
Concept introduction:
The electron dot formula is used for the identification of valence electrons present in an atom. It shows the symbol of an atom with its valence electron around it. It is also called as Lewis dot structure. The valence electrons around an atom are shown by dots. One dot represents one valence electron.
The rules to draw electron dot formula are given below.
• First, the symbol of an element is written.
• With the help of group number, the number of valence electrons is determined.
• The valance electrons are drawn around the atom by dots.
Answer to Problem 62E
The electron dot structure for the atom of
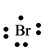
Explanation of Solution
Bromine belongs to the group
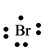
Figure 8
The electron dot structure of
Want to see more full solutions like this?
Chapter 5 Solutions
EP INTRODUCTORY CHEM.-MOD.MASTERINGCHEM
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardCan you please help me and explain how I would find a mechanism consistent, using my results. Help with number 5.arrow_forwardThe conversion of (CH3)3CI to (CH3)2C=CH2 can occur by either a one-step or a two-step mechanism, as shown in Equations [1] and [2]. [1] + I + H₂Ö: :OH [2] q slow :OH + I¯ H₂Ö: a. What rate equation would be observed for the mechanism in Equation [1]? b. What rate equation would be observed for the mechanism in Equation [2]? c. What is the order of each rate equation (i.e., first, second, and so forth)? d. How can these rate equations be used to show which mechanism is the right one for this reaction? e. Assume Equation [1] represents an endothermic reaction and draw an energy diagram for the reaction. Label the axes, reactants, products, Ea, and AH°. Draw the structure for the transition state. f. Assume Equation [2] represents an endothermic reaction and that the product of the rate-determining step is higher in energy than the reactants or products. Draw an energy diagram for this two-step reaction. Label the axes, reactants and products for each step, and the Ea and AH° for each…arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
- For a complex reaction with the rate equation v = k1[A] + k2[A]2, we can say(A) that it is of order 1.(B) that it is of order 1.5.(C) that it is of order 2.(D) that for certain values of [A] it can behave as if it were of order 1, and for other values as if it were of order 2.arrow_forwarda. Draw a complete arrow pushing mechanism for the following. Is this the thermodynamic or the kinetic product? Use your mechanism to explain your choice. Draw all the resonance. HBr Brarrow_forwardWhich, if any, of the substances had resonance structures? How many resonance structures did each substance have from the following list: CCl4 H2O CO2 C2H4 NH3 SF6 ICl5arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning


