
Concept explainers
(a)
Interpretation:
The specific relationship between the pair of molecules shown is to be determined.
Concept introduction:
The intersection of a horizontal and vertical line indicates a carbon atom- typically an asymmetric carbon. The atom at the intersection is a carbon atom. The substituents on the vertical bonds point away from the observer while those on the horizontal bonds point toward the observer.
To determine the R/S configuration at the asymmetric carbon (chiral center), the vertical bonds are replaced by dash bonds and horizontal ones by wedge bonds. The groups on these bonds are then assigned priorities according to Cahn-Ingold-Prelog rules. If the priority groups 1 to 3 are arranged clockwise with the lowest priority group pointing away (at the back), the configuration of the asymmetric carbon is R. If they are arranged counterclockwise, the configuration is S.
If the lowest priority group is pointing toward the observer (it is in the front), then the actual configuration is reverse of that indicated. That means if the arrangement is clockwise, the actual configuration is S, and if counterclockwise, it is R.
If the molecules have different formulas, they are unrelated compounds. If the formulas are same, but the connectivity of atoms is different, the molecules are constitutional isomers.
If the formulas as well as connectivities are the same, then the molecules may be the same or stereoisomers.
The configuration of each asymmetric carbon is then compared to determine the specific relationship between the two molecules. If the configurations are the same, the molecules may be the same or conformers of the same molecule. Conformers are molecules that can be interconverted by a rotation about a single bond.
If the configurations at a single chiral center are different, then the two molecules are enantiomers.
If the molecule contains more than one chiral center, then a difference in the configuration of all chiral centers makes them enantiomers. If the configurations are different at some but not all the chiral centers, then the molecules are diastereomers.
Answer to Problem 5.73P
The molecules are the same.
Explanation of Solution
The Fischer projections are
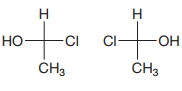
After converting the Fischer projections to dash-wedge representation, it can be shown that the two are the same.
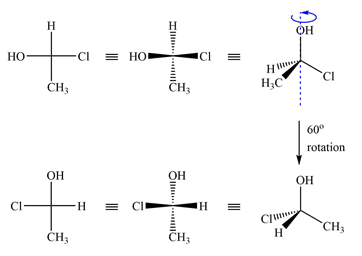
Rotating the first molecule through
Therefore, the two molecules are the same.
The two molecules are identical because rotating the dash-wedge structures interconverts them into each other.
(b)
Interpretation:
The specific relationship between the pair of molecules shown is to be determined.
Concept introduction:
The intersection of a horizontal and vertical line indicates a carbon atom- typically an asymmetric carbon. The atom at the intersection is a carbon atom. The substituents on the vertical bonds point away from the observer while those on the horizontal bonds point toward the observer.
To determine the R/S configuration at the asymmetric carbon (chiral center), the vertical bonds are replaced by dash bonds and horizontal ones by wedge bonds. The groups on these bonds are then assigned priorities according to Cahn-Ingold-Prelog rules. If the priority groups 1 to 3 are arranged clockwise with the lowest priority group pointing away (at the back), the configuration of the asymmetric carbon is R. If they are arranged counterclockwise, the configuration is S.
If the lowest priority group is pointing toward the observer (it is in the front), then the actual configuration is reverse of that indicated. That means if the arrangement is clockwise, the actual configuration is S, and if counterclockwise, it is R.
If the molecules have different formulas, they are unrelated compounds. If the formulas are same, but the connectivity of atoms is different, the molecules are constitutional isomers. If the formulas as well as connectivities are the same, then the molecules may be the same or stereoisomers.
The configuration of each asymmetric carbon is then compared to determine the specific relationship between the two molecules. If the configurations are the same, the molecules may be the same or conformers of the same molecule. Conformers are molecules that can be interconverted by a rotation about a single bond.
If the configurations at a single chiral center are different, then the two molecules are enantiomers.
If the molecule contains more than one chiral center, then a difference in the configuration of all chiral centers makes them enantiomers. If the configurations are different at some but not all the chiral centers, then the molecules are diastereomers.
Answer to Problem 5.73P
The two molecules are the same.
Explanation of Solution
The Fischer projections are
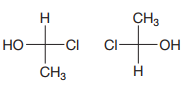
The first projection is converted into the second one by a simple rotation through
Rotating a Fischer projection in the plane of the paper does not change the configuration.
(c)
Interpretation:
The specific relationship between the pair of molecules shown is to be determined.
Concept introduction:
The intersection of a horizontal and vertical line indicates a carbon atom- typically an asymmetric carbon. The atom at the intersection is a carbon atom. The substituents on the vertical bonds point away from the observer while those on the horizontal bonds point toward the observer.
To determine the R/S configuration at the asymmetric carbon (chiral center), the vertical bonds are replaced by dash bonds and horizontal ones by wedge bonds. The groups on these bonds are then assigned priorities according to Cahn-Ingold-Prelog rules. If the priority groups 1 to 3 are arranged clockwise with the lowest priority group pointing away (at the back), the configuration of the asymmetric carbon is R. If they are arranged counterclockwise, the configuration is S.
If the lowest priority group is pointing toward the observer (it is in the front), then the actual configuration is reverse of that indicated. That means if the arrangement is clockwise, the actual configuration is S, and if counterclockwise, it is R.
If the molecules have different formulas, they are unrelated compounds. If the formulas are same, but the connectivity of atoms is different, the molecules are constitutional isomers.
If the formulas as well as connectivities are the same, then the molecules may be the same or stereoisomers.
The configuration of each asymmetric carbon is then compared to determine the specific relationship between the two molecules. If the configurations are the same, the molecules may be the same or conformers of the same molecule. Conformers are molecules that can be interconverted by a rotation about a single bond.
If the configurations at a single chiral center are different, then the two molecules are enantiomers.
If the molecule contains more than one chiral center, then a difference in the configuration of all chiral centers makes them enantiomers. If the configurations are different at some but not all the chiral centers, then the molecules are diastereomers.
Answer to Problem 5.73P
The two molecules are enantiomers.
Explanation of Solution
The Fischer projections are
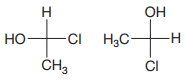
The projections are converted to sash-wedge representations as shown below. The four groups on the chiral carbon are then assigned priorities according to Cahn-Ingold-Prelog rules.
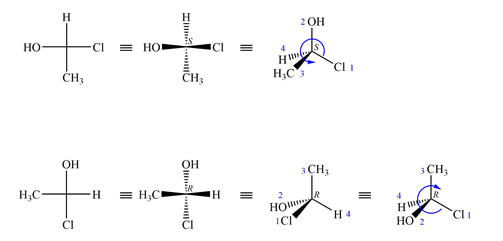
The priority 1 to 3 groups in the first molecule are arranged counterclockwise with the lowest priority H at the back. The configuration at this carbon is then S.
In the second molecule, these groups are arranged counterclockwise; therefore, its configuration is R, opposite that of the first molecule.
Therefore, the two molecules are enantiomers.
Molecules with a single chiral center are enantiomers if their configurations are different.
(d)
Interpretation:
The specific relationship between the pair of molecules shown is to be determined.
Concept introduction:
The intersection of a horizontal and vertical line indicates a carbon atom- typically an asymmetric carbon. The atom at the intersection is a carbon atom. The substituents on the vertical bonds point away from the observer while those on the horizontal bonds point toward the observer.
To determine the R/S configuration at the asymmetric carbon (chiral center), the vertical bonds are replaced by dash bonds and horizontal ones by wedge bonds. The groups on these bonds are then assigned priorities according to Cahn-Ingold-Prelog rules. If the priority groups 1 to 3 are arranged clockwise with the lowest priority group pointing away (at the back), the configuration of the asymmetric carbon is R. If they are arranged counterclockwise, the configuration is S.
If the lowest priority group is pointing toward the observer (it is in the front), then the actual configuration is reverse of that indicated. That means if the arrangement is clockwise, the actual configuration is S, and if counterclockwise, it is R.
If the molecules have different formulas, they are unrelated compounds. If the formulas are same, but the connectivity of atoms is different, the molecules are constitutional isomers.
If the formulas as well as connectivities are the same, then the molecules may be the same or stereoisomers.
The configuration of each asymmetric carbon is then compared to determine the specific relationship between the two molecules. If the configurations are the same, the molecules may be the same or conformers of the same molecule. Conformers are molecules that can be interconverted by a rotation about a single bond.
If the configurations at a single chiral center are different, then the two molecules are enantiomers.
If the molecule contains more than one chiral center, then a difference in the configuration of all chiral centers makes them enantiomers. If the configurations are different at some but not all the chiral centers, then the molecules are diastereomers.
Answer to Problem 5.73P
The two molecules are enantiomers.
Explanation of Solution
The Fischer projections of the molecules are
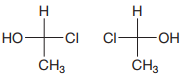
The molecules can be interconverted by exchanging the positions of two of the four groups,
Therefore these are enantiomer.
Exchanging positions of two of the four groups attached to an asymmetric carbon inverts its configuration.
(e)
Interpretation:
The specific relationship between the pair of molecules shown is to be determined.
Concept introduction:
The intersection of a horizontal and vertical line indicates a carbon atom- typically an asymmetric carbon. The atom at the intersection is a carbon atom. The substituents on the vertical bonds point away from the observer while those on the horizontal bonds point toward the observer.
To determine the R/S configuration at the asymmetric carbon (chiral center), the vertical bonds are replaced by dash bonds and horizontal ones by wedge bonds. The groups on these bonds are then assigned priorities according to Cahn-Ingold-Prelog rules. If the priority groups 1 to 3 are arranged clockwise with the lowest priority group pointing away (at the back), the configuration of the asymmetric carbon is R. If they are arranged counterclockwise, the configuration is S.
If the lowest priority group is pointing toward the observer (it is in the front), then the actual configuration is reverse of that indicated. That means if the arrangement is clockwise, the actual configuration is S, and if counterclockwise, it is R.
If the molecules have different formulas, they are unrelated compounds. If the formulas are same, but the connectivity of atoms is different, the molecules are constitutional isomers.
If the formulas as well as connectivities are the same, then the molecules may be the same or stereoisomers.
The configuration of each asymmetric carbon is then compared to determine the specific relationship between the two molecules. If the configurations are the same, the molecules may be the same or conformers of the same molecule. Conformers are molecules that can be interconverted by a rotation about a single bond.
If the configurations at a single chiral center are different, then the two molecules are enantiomers.
If the molecule contains more than one chiral center, then a difference in the configuration of all chiral centers makes them enantiomers. If the configurations are different at some but not all the chiral centers, then the molecules are diastereomers.
Answer to Problem 5.73P
The two molecules are enantiomers.
Explanation of Solution
The Fischer projections of the two molecules are
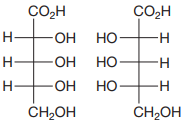
The molecules are aldonic acids with the same molecular formula and connectivity. Each contains three asymmetric carbons, C2, C3, and C4. Therefore, they can be the same molecules, conformers, or configurational isomers.
The two cannot be interconverted by a rotation about a single bond; therefore, they are not conformers.
The configurations of each of these three must be compared to determine the specific relation between the two molecules.
To determine the configurations of the three asymmetric carbons, the Fischer projection is redrawn as a dash-wedge structure at each carbon.
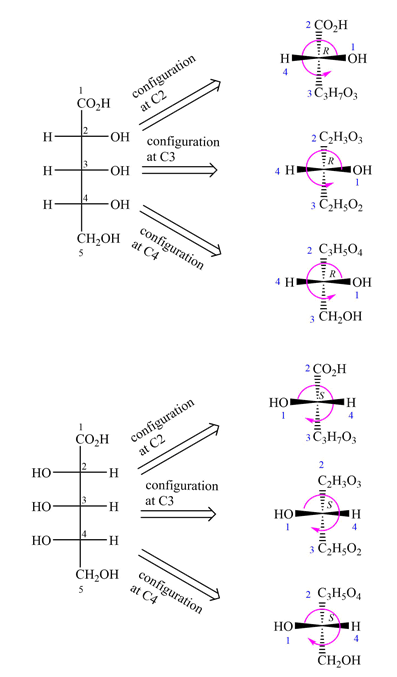
The vertical bonds in the Fischer projection are pointed away from the observer, while the horizontal ones are pointed toward the observer. The four groups attached to the asymmetric carbon are then assigned priorities according to Cahn-Ingold-Prelog rules. Based on the priorities, the configurations at the three asymmetric carbons in the first molecule are
Therefore, the two molecules are enantiomers.
When the configurations at every asymmetric carbon in the pair of molecules with the same formula and connectivity are different, the molecules are enantiomers.
(f)
Interpretation:
The specific relationship between the pair of molecules shown is to be determined.
Concept introduction:
The intersection of a horizontal and vertical line indicates a carbon atom- typically an asymmetric carbon. The atom at the intersection is a carbon atom. The substituents on the vertical bonds point away from the observer while those on the horizontal bonds point toward the observer.
To determine the R/S configuration at the asymmetric carbon (chiral center), the vertical bonds are replaced by dash bonds and horizontal ones by wedge bonds. The groups on these bonds are then assigned priorities according to Cahn-Ingold-Prelog rules. If the priority groups 1 to 3 are arranged clockwise with the lowest priority group pointing away (at the back), the configuration of the asymmetric carbon is R. If they are arranged counterclockwise, the configuration is S.
If the lowest priority group is pointing toward the observer (it is in the front), then the actual configuration is reverse of that indicated. That means if the arrangement is clockwise, the actual configuration is S, and if counterclockwise, it is R.
If the molecules have different formulas, they are unrelated compounds. If the formulas are same, but the connectivity of atoms is different, the molecules are constitutional isomers.
If the formulas as well as connectivities are the same, then the molecules may be the same or stereoisomers.
The configuration of each asymmetric carbon is then compared to determine the specific relationship between the two molecules. If the configurations are the same, the molecules may be the same or conformers of the same molecule. Conformers are molecules that can be interconverted by a rotation about a single bond.
If the configurations at a single chiral center are different, then the two molecules are enantiomers.
If the molecule contains more than one chiral center, then a difference in the configuration of all chiral centers makes them enantiomers. If the configurations are different at some but not all the chiral centers, then the molecules are diastereomers.
Answer to Problem 5.73P
The molecules are diastereomers.
Explanation of Solution
The Fischer projections of the two molecules are
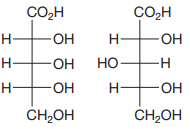
The molecules are aldonic acids with the same molecular formula and connectivity. Each contains three asymmetric carbons, C2, C3, and C4. Therefore, they can be the same molecules, conformers, or configurational isomers.
The two cannot be interconverted by a rotation about a single bond; therefore, they are not conformers.
The configurations of each of these three must be compared to determine the specific relation between the two molecules.
To determine the configurations of the three asymmetric carbons, the Fischer projection is redrawn as a dash-wedge structure at each carbon.
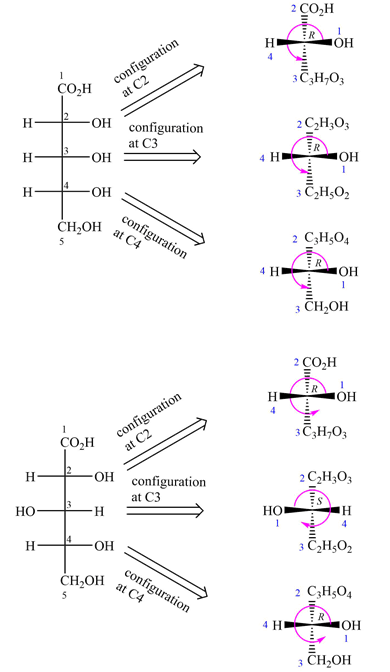
The vertical bonds in the Fischer projection are pointed away from the observer while the horizontal ones are pointed toward the observer. The four groups attached to the asymmetric carbon are then assigned priorities according to Cahn-Ingold-Prelog rules. Based on the priorities, the configurations at the three asymmetric carbons in the first molecule are
Therefore, the two molecules are diastereomers.
Two chiral molecules with multiple chiral centers that differ in configuration at some but not all chiral centers are diastereomers.
Want to see more full solutions like this?
Chapter 5 Solutions
ORG CHEM W/ EBOOK & SW5 + STUDY GUIDE
- B 1 of 2 Additional problems in preparation to Midterm #1: 1.) How can the following compounds be prepared using Diels-Alder reaction: CH3 O CN (a) (b) CN CH3 2.) What is the missing reagent in the shown reaction? H3C + ? H3C H3C CN H3C ''CN (၁) H 3.) Write the products 1,2-addition and 1,4-addition of DBr to 1,3-cyclohexadiene. Remember, D is deuterium, a heavy isotope of hydrogen. It reacts exactly like hydrogen. 4.) In the shown reaction, which will be the kinetic product and which will be the thermodynamic product? H3C CI H3C HCI H3C + 5.) Which of the following molecules is aromatic? (a) (b) (c) H 6.) Which of the following molecules is aromatic? (a) (b) (c) 7.) Write the mechanism for the shown reaction. + Ха AICI 3 CI 8.) Suggest reagents that would convert benzene into the shown compounds. CI NO2 -8-6-6-8-a (a) (b) (c) (d) (e) (a) SO3H Brarrow_forwardThe number of 2sp^2 hybridized atoms in is: A. 8; B. 6; C.4; D.2; E.0;arrow_forwardThe highest boiling compound from among the following isA. 2-methylheptane; B. 3-methylheptane; C. 2,2-dimethylhexane;D. octane; E. 2,2,3-trimethylpentanearrow_forward
- Which of the following features are found in the most stable structure ofCH5NO that does not have a CO bond?w. a π bond, x. two NH bonds, y. one OH bond, z. 3 lone pairsA. w, x; B. x, y; C. y, z; D. x, y, z; E. all of them.arrow_forwardWhich one of the following functional groups is not present in thecompound shownA. amine; B. aldehyde, C. ether; D. amide. E. ketonearrow_forwardWhich of the following formulas correspond to at least one compound inwhich resonance is important?w. C2H5N x. C3H5Br; y. C3H4; z. C4H6.A. w, x, y; B. x, y, z; C. w, x, z; D. w, y, z; E. all of themarrow_forward
- Predict the product(s) that are formed after each step for reactions 1-4. In each case, consider formation of any chiral center(s) and draw all expected stereoisomers. 1) OH 1) HBr (SN2) 2) NaOH, heat 3) BH3, THF 4) H2O2, NaOH 2) OH 1) SOCI 2, py 2) NaOEt 3) Br2, H₂O 3) OH 1) H2SO4 conc. 2) HBr, ROOR 3) KOtBu 4) OH 1) TsCl, py 2) NaOEt 3) 03 4) DMSarrow_forwardWhich of the following rings has the least strain in its most stableconformation?A. Cyclobutane; B. Cyclopentane; C. Cyclohexane; D. Cycloheptane;E. Cyclooctanearrow_forwardThe number of different carbon skeletons that have a main chain of 9carbons and an ethyl branch isA 3; B. 4; C. 5; D. 6; E. 7arrow_forward
- Q5: Classify the following pair of molecules as constitutional isomers, enantiomers, diastereomers, the same molecule, or completely different molecules. Br O CI Br OH OH 111 Br .!!!/Br F OH and ...m Br Br OH CI Br OH ་་་་་" ། ་arrow_forwardConsidering only rotation around the 1-2 bond, how many differentstaggered conformations are there of 1,2-dibromo-1,2-dichloropropane?A: 2; B. 3; C. 4; D. 6; E. more than 6.arrow_forwardcan you help me solve thisarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
