
Concept explainers
(a)
Interpretation:
Whether the compounds in the given pair have the same or different boiling points is to be determined.
Concept introduction:
Isomers are the pair of compounds that have the same formula.
Constitutional isomers are the isomers having the same molecular formula but different connectivity. Constitutional isomers must have different physical and chemical properties.
The configurational isomers are not interconvertible by rotating around a single bond.
Enantiomers are configurational isomers having the same connectivity but cannot be interconvertible by rotation around a single bond. The mirror images of enantiomers are non-superimposable. Since enantiomers have the same connectivity of atoms, they should behave identically.
Diastereomers are configurational isomers having same connectivity but are not mirror images of each other. Diastereomers show different physical and chemical properties. Cis-trans isomers are diastereomers.
The boiling point of isomers comes under physical property.
Answer to Problem 5.53P
The given pair of isomers have different boiling points as they are diastereomers of each other.
Explanation of Solution
The given pair of compounds is
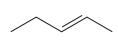
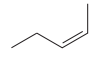
Both the molecules in the given pair have the same molecular formula; they also have the same connectivity. Thus, they are configurational isomers. The given pair of molecules is cis-trans isomers. So, these compounds are diastereomers of each other. Diastereomers show different physical and chemical properties. Thus, the compounds in the given pair should have different boiling points.
The given pair of isomers have different boiling point as they are diastereomers of each other.
(b)
Interpretation:
Whether the compounds in the given pair have the same or different boiling points is to be determined.
Concept introduction:
Isomers are the pair of compounds that have the same formula.
Constitutional isomers are the isomers having the same molecular formula but different connectivity. Constitutional isomers must have different physical and chemical properties.
The configurational isomers are not interconvertible by rotating around a single bond.
Enantiomers are configurational isomers having the same connectivity but cannot be interconvertible by rotation around a single bond. The mirror images of enantiomers are non-superimposable. Since enantiomers have the same connectivity of atoms, they should behave identically.
Diastereomers are configurational isomers that have the same connectivity but are not mirror images of each other. Diastereomers have different physical and chemical properties. Cis-trans isomers are diastereomers of each other.
The boiling point of isomers comes under physical property.
Answer to Problem 5.53P
The given pair of isomers have different boiling points as they are constitutional isomers of each other.
Explanation of Solution
The given pair of compounds is
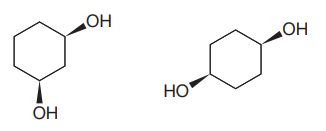
Both the molecules in the given pair have the same molecular formula, so they are isomers.
However, they do not have same connectivity of atoms. In both the compounds, there are two
In the first compound, the
The given pair of isomers have different boiling points as they are constitutional isomers of each other.
(c)
Interpretation:
Whether the compounds in the given pair have the same or different boiling points is to be determined.
Concept introduction:
Isomers are the pair of compounds that have the same formula.
Constitutional isomers are the isomers having the same molecular formula but different connectivity. Constitutional isomers must have different physical and chemical properties.
The configurational isomers are not interconvertible by rotating around a single bond.
Enantiomers are configurational isomers having the same connectivity but cannot be interconvertible by rotation around a single bond. The mirror images of enantiomers are non-superimposable. Since enantiomers have the same connectivity of atoms, they should behave identically.
Diastereomers are configurational isomers that have the same connectivity but are not mirror images of each other. Diastereomers have different physical and chemical properties. Cis-trans isomers are diastereomers of each other.
The boiling point of isomers comes under physical property.
In a Fischer projection, exchanging two groups on an asymmetric carbon atoms gives the opposite stereochemical configuration.
Answer to Problem 5.53P
The given pair of isomers should have different boiling points as they are diastereomers of each other.
Explanation of Solution
The given pair of compounds is
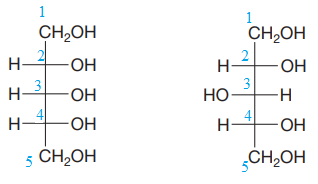
Both the molecules in the given pair have the same molecular formula. They also have the same connectivity. Thus, they are configurational isomers.
Each molecule has three chiral centers at C2, C3, and C4 carbon atoms. Note that in the second compound, the two groups on C3 chiral carbon have been exchanged. This suggests that the stereochemical configurations at C3 carbon atoms in both the compounds are opposite to each other. Remaining stereo centers have the same stereochemical configuration, suggesting that the two compounds are diastereomers of each other. Diastereomers have different physical and chemical properties. Thus, the compounds in the given pair should have different boiling points.
The given pair of isomers have different boiling points as they are diastereomers of each other.
(d)
Interpretation:
Whether the compounds in the given pair have the same or different boiling points is to be determined.
Concept introduction:
Isomers are the pair of compounds that have the same formula.
Constitutional isomers are the isomers having the same molecular formula but different connectivity. Constitutional isomers must have different physical and chemical properties.
The configurational isomers are not interconvertible by rotating around a single bond.
Enantiomers are configurational isomers having the same connectivity but cannot be interconvertible by rotation around a single bond. The mirror images of enantiomers are non-superimposable. Since enantiomers have the same connectivity of atoms, they should behave identically.
Diastereomers are configurational isomers that have the same connectivity but are not mirror images of each other. Diastereomers have different physical and chemical properties. Cis-trans isomers are diastereomers of each other. Cyclic
The boiling point of isomers comes under physical property.
Answer to Problem 5.53P
The given pair of isomers should have different boiling points as they are diastereomers of each other.
Explanation of Solution
The given pair of compounds is
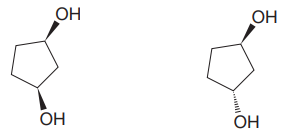
In both the compounds, the
The given pair of isomers have different boiling points as they are diastereomers of each other.
(e)
Interpretation:
Whether the compounds in the given pair have the same or different boiling points is to be determined.
Concept introduction:
Isomers are the pair of compounds that have the same formula.
Constitutional isomers are the isomers having the same molecular formula but different connectivity. Constitutional isomers must have different physical and chemical properties.
The configurational isomers are not interconvertible by rotating around a single bond.
Enantiomers are configurational isomers having the same connectivity but cannot be interconvertible by rotation around a single bond. The mirror images of enantiomers are non-superimposable. Since enantiomers have the same connectivity of atoms, they should behave identically.
The boiling point of isomers comes under physical property.
Answer to Problem 5.53P
The given pair of isomers should have the same boiling point as they are enantiomers of each other.
Explanation of Solution
The given pair of compounds is
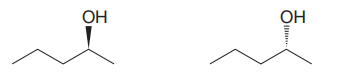
In both the compounds, the
The stereochemical configuration at the chiral center in the first molecule is S as the top-three priority groups are arranged in a counterclockwise manner, and the fourth-priority group is on a dash bond.
The stereochemical configuration at the chiral center in the second molecule is R as the top-three priority groups are arranged in a counterclockwise manner, but the fourth-priority group is on a wedge bond.
Thus, the stereochemical configuration at the chiral centers for two molecules is opposite. This indicates that the two compounds must be enantiomers of each other. Enantiomers have precisely the same physical and chemical properties. Hence the two compounds in the given pair have the same boiling points.
The given pair of isomers should have the same boiling point as they are enantiomers of each other.
Want to see more full solutions like this?
Chapter 5 Solutions
ORG CHEM W/ EBOOK & SW5 + STUDY GUIDE
- Steps and explanations pleasearrow_forwardUse diagram to answer the following: 1.Is the overall rxn endo- or exothermic. Explain briefly your answer____________________2. How many steps in this mechanism?_____________3. Which is the rate determining step? Explain briefly your answer____________________4. Identify (circle and label) the reactants,the products and intermediate (Is a Cation, Anion, or a Radical?) Please explain and provide full understanding.arrow_forwardDraw the entire mechanism and add Curved Arrows to show clearly how electrons areredistributed in the process. Please explain and provide steps clearly.arrow_forward
- Match the denticity to the ligand. Water monodentate ✓ C₂O2 bidentate H₂NCH₂NHCH2NH2 bidentate x EDTA hexadentate Question 12 Partially correct Mark 2 out of 2 Flag question Provide the required information for the coordination compound shown below: Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2✔ Geometry: linear Oxidation state of transition metal ion: +3 x in 12 correct out of 2 question Provide the required information for the coordination compound shown below. Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2 Geometry: linear 0 Oxidation state of transition metal ion: +3Xarrow_forwardCan you explain step by step behind what the synthetic strategy would be?arrow_forwardPlease explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward
- 2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forwardconsider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning




