
ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259977596
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 5.59P
Explain each statement by referring to compounds A-E.
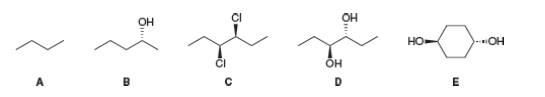
a. A has a mirror image bur no enantiomer.
b. B has an enantiomer and no diastereomer.
c. C has both an enantiomer and a diastereomer.
d. D has a diastereomer but no enantiomer.
e. E has a diastereomer but no enantiomer.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used hand raiting
A vial of Xe 133 gas (t 1/2 = 5.24 d) os ca;obrated fpr 22mCi @ 6:00am on March 1. What is its activity at 6:00 pm on march 8? what is mCI remain
McLafferty Rearrangement: Label alpha (), beta (), and gamma () on the molecule. Draw mechanismarrows to describe the process of the rearrangement. What functional group is lost during the rearrangement? What new functional group is made from the ketone/aldehyde you started with? What stabilizing chemical theory causes (allows) rearrangement to happen?
Chapter 5 Solutions
ORGANIC CHEMISTRY
Ch. 5 - Prob. 5.1PCh. 5 - Prob. 5.2PCh. 5 - Draw the mirror image of each compound. Label each...Ch. 5 - Prob. 5.4PCh. 5 - A molecule is achiral if it has a plane of...Ch. 5 - Problem 5.6 Locate the stereogenic centers in each...Ch. 5 - The facts in section 5.4A can be used to locate...Ch. 5 - Prob. 5.8PCh. 5 - Prob. 5.9PCh. 5 - Prob. 5.10P
Ch. 5 - Prob. 5.11PCh. 5 - Prob. 5.12PCh. 5 - Label each compound as R or S.Ch. 5 - Prob. 5.14PCh. 5 - Prob. 5.15PCh. 5 - Prob. 5.16PCh. 5 - Prob. 5.17PCh. 5 - Problem 5.18 Compounds E and F are two isomers of...Ch. 5 - Prob. 5.19PCh. 5 - Prob. 5.20PCh. 5 - Prob. 5.21PCh. 5 - Prob. 5.22PCh. 5 - Prob. 5.23PCh. 5 - Prob. 5.24PCh. 5 - Which of the following cyclic molecules are meso...Ch. 5 - Prob. 5.26PCh. 5 - Prob. 5.27PCh. 5 - Problem 5.28 The amino acid has the physical...Ch. 5 - Prob. 5.29PCh. 5 - Prob. 5.30PCh. 5 - Prob. 5.31PCh. 5 - Prob. 5.32PCh. 5 - Prob. 5.33PCh. 5 - Prob. 5.34PCh. 5 - Prob. 5.35PCh. 5 - Prob. 5.36PCh. 5 - Prob. 5.37PCh. 5 - Prob. 5.38PCh. 5 - Prob. 5.39PCh. 5 - 5.40 Determine if each compound is identical to or...Ch. 5 - Prob. 5.41PCh. 5 - Prob. 5.42PCh. 5 - Prob. 5.43PCh. 5 - Prob. 5.44PCh. 5 - Prob. 5.45PCh. 5 - Prob. 5.46PCh. 5 - Label each stereogenic center as R or S. a. c. e....Ch. 5 - Prob. 5.48PCh. 5 - Prob. 5.49PCh. 5 - Prob. 5.50PCh. 5 - Prob. 5.51PCh. 5 - Prob. 5.52PCh. 5 - Prob. 5.53PCh. 5 - Prob. 5.54PCh. 5 - Prob. 5.55PCh. 5 - Prob. 5.56PCh. 5 - Prob. 5.57PCh. 5 - Prob. 5.58PCh. 5 - 5.59 Explain each statement by referring to...Ch. 5 - Prob. 5.60PCh. 5 - Prob. 5.61PCh. 5 - Prob. 5.62PCh. 5 - Prob. 5.63PCh. 5 - Prob. 5.64PCh. 5 - Prob. 5.65PCh. 5 - Prob. 5.66PCh. 5 -
5.67 Artemisinin and mefloquine are widely used...Ch. 5 - 5.68 Saquinavir (trade name Invirase) is a...Ch. 5 - Prob. 5.69PCh. 5 - Prob. 5.70PCh. 5 - Prob. 5.71PCh. 5 - Prob. 5.72PCh. 5 - Problem 5.73 An acid-base reaction of with a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
Some people compare DNA to a blueprint stored in the office of a construction company. Explain how this analogy...
Biology: Concepts and Investigations
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
What process causes the Mediterranean intermediate Water MIW to become more dense than water in the adjacent At...
Applications and Investigations in Earth Science (9th Edition)
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't used hand raitingarrow_forwardDon't used hand raitingarrow_forwardIf a high molecular weight linear polyethylene is chlorinated by inducing the substitution of chlorine atoms by hydrogen, if 5% of all hydrogen atoms are replaced, what approximate percentage of chlorine by weight would the product have?arrow_forward
- O Macmillan Learning Chemistry: Fundamentals and Principles Davidson presented by Macmillan Learning Poly(ethylene terephthalate), known as PET or industrially as Dacron, is a polyester synthesized through a condensation reaction between two bifunctional monomers. The monomers, ethylene glycol and terepthalic acid, are given. Add bonds and remove atoms as necessary to show the structure of a two repeat unit portion of a longer polymer chain of PET. You may need to zoom out to see the complete structure of all four monomer units. Select Draw / || | C H 0 3 © Templates More ° ° ° || C CC - OH HO OH HOC - C Erase CC OH HO C C 〃 C H₂ Q2Qarrow_forwardc) + H₂Oarrow_forward으 b) + BF. 3 H2Oarrow_forward
- Q4: Draw the product of each Lewis acid-bas reaction. Label the electrophile and nucleophile. b) S + AICI 3 + BF 3arrow_forwardQ1 - What type(s) of bonding would be expected for each of the following materials: solid xenon, calcium fluoride (CaF2), bronze, cadmium telluride (CdTe), rubber, and tungsten? Material solid xenon CaF2 bronze CdTe rubber tungsten Type(s) of bonding Q2- If the atomic radius of lead is 0.175 nm, calculate the volume of its unit cell in cubic meters.arrow_forwardDetermine the atomic packing factor of quartz, knowing that the number of Si atoms per cm3 is 2.66·1022 and that the atomic radii of silicon and oxygen are 0.038 and 0.117 nm.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License